Prequalification Procedures and Fees: Immunization Devices
Who can participate in WHO prequalification of immunization devices?
Any manufacturer of an immunization-related product or device (hereafter, "product") belonging to a category that WHO prequalifies can submit a product for prequalification. Specifically, WHO prequalifies products that are offered by the legal manufacturer of the product. Products offered by a reseller may also be considered for prequalification if a formal licensing arrangement has been made with the legal manufacturer.
Manufacturers should be aware that WHO IMD-PQS only convenes with holders of IMD-PQS prequalification (manufacturers or resellers of products that have been granted prequalified status by WHO Immunization Devices prequalification. Manufacturers or resellers interested in pursuing prequalification should begin by reviewing the relevant IMD-PQS product specification(s) and, if appropriate, by submitting a pre-submission form (by email, to the IMD-PQS Secretariat).
What categories of products can be prequalified?
WHO prequalifies products and devices belonging to the following categories:
- E001: Cold rooms, freezer rooms, and related equipment
- E002: Refrigerated vehicles
- E003: Refrigerators and freezers
- E004: Cold boxes and vaccine carriers
- E005: Coolant-packs and vaccine carriers
- E006: Cold chain accessories
- E007: Cold chain accessories
- E008: Injection devices for immunization
- E010: Waste management equipment
- E013: Injection devices for therapeutic purposes
Products in any of these categories may be submitted for prequalification.
Developers and manufacturers of product in these categories might also wish to refer to the WHO target product profiles (TPPs). TPPs describe desired future features and characteristics that will, over time, become incorporated into product specifications as requirements.
2026 Pipeline survey
In order to facilitate the efficient prequalification of immunization devices, and to ensure visibility over the pipeline of devices that may be available for WHO immunization programmes, WHO IMD-PQS kindly requests current and potential prequalification holders to complete this online pipeline survey, indicating the applications they expect to submit over the course of 2026.
How are products prequalified?
The full product prequalification procedure consists of three stages:
- completion of a pre-submission form
- prequalification application dossier submission, evaluation (via the WHO's e-Prequalification platform) and product testing
- annual post-prequalification committments to ensure that the prequalified product continues to comply with WHO prequalification requirements.
General application timelines
- Pre-submission: IMD-PQS will contact the manufacturer within 30 days of receiving a complete pre-submission form.
- Dossier review and decision: IMD-PQS will contact the manufacturer within 60 days of receiving the dossier depending on the number of clarification questions raised by the dossier review team (with the exception of category E001, for which IMD-PQS will respond within 90 days). Response time excludes wait-times whilst applicants are gathering and submitting the requested additional information.
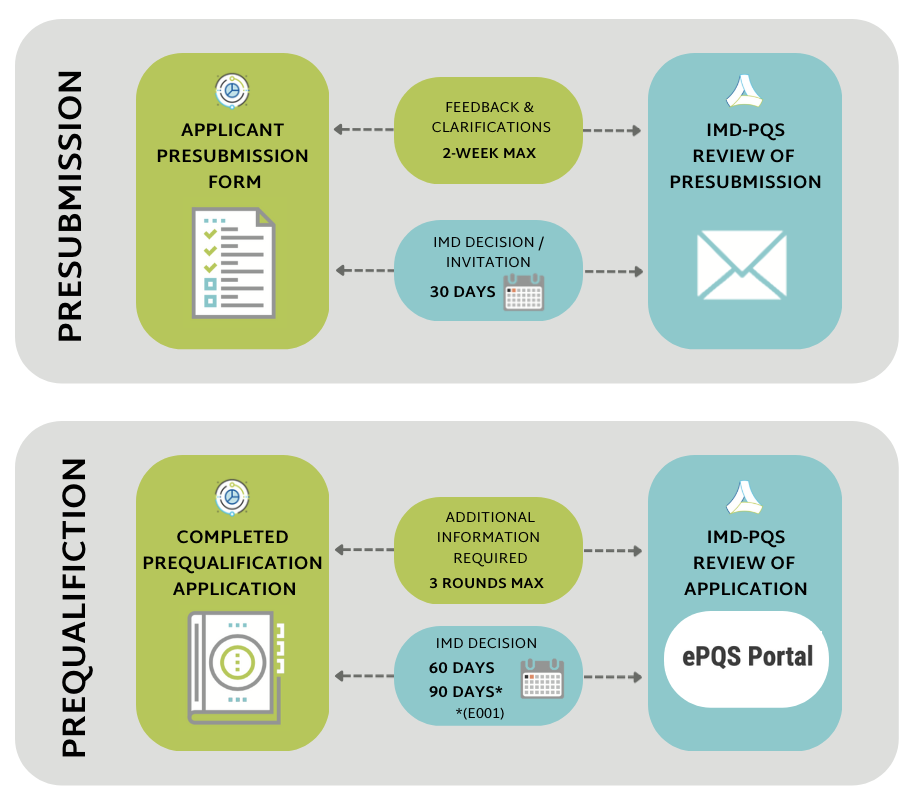
IMPORTANT: IMD-PQS response times depends on the speed and completeness of applicant replies to IMD-PQS requests for further documents or information: response time excludes wait-times whilst applicants are gathering and submitting the requested additional information.
Manufacturers may also find it useful to consult the relevant IMD-PQS standard operating procedures (SOPs).
Prequalification fees
A complete list of fees due as a part of the prequalification process and post-prequalification commitments is available on the page "Cost of prequalification".
Three different fees will be due over the lifetime of a WHO IMD-PQS prequalified product:
- Prequalification application fee
- Laboratory testing fees
- Annual Review of prequalified products
Inspections of a Prequalification Holder's manufacturing site may, in some cases, be required as a part of post-prequalification obligations. In such cases, inspections fees will be due on a cost recovery basis.
