Pre-submission procedure
Pre-submission form (screening)
Prospective Prequalification Holders who wish to submit a product for prequalification must begin the application process by submitting a complete pre-submission form and additional required documentation. A product manufacturer or licenced reseller should refer to the relevant IMD-PQS performance specification and verification protocol(s) to assess whether their product corresponds to an IMD-PQS product category and whether a pre-submission is warranted.
The prequalification Pre-submission form is available here.

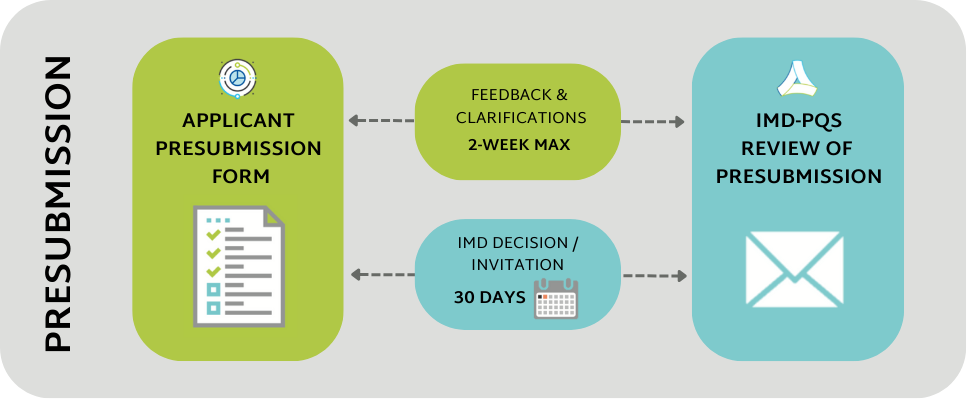
The purpose of the pre-submission stage is to screen potential applications for prequalification to establish whether they correspond to an active IMD-PQS product category, i.e. that answers the needs of national immunization programmes. Pre-submission screening also allows IMD-PQS to identify whether a proposed product is likely to meet the programme's performance, quality and safety criteria. The information in the pre-submission form will assist WHO in determining whether the product is eligible for WHO prequalification assessment.
Pre-submission required documentation
The pre-submission screening process requires applicants to provide the following information and documentation:
- Applicant company information
- Authorised contacts for the applicant
- Product name & manufacturer product reference
- Name and number of the reference IMD-PQS product specification
- Previous product testing information
- Licensing information and documents
- Certification information and documents
- WHO history of product
- Prospective Prequalification Holder declaration
All documents submitted as a part of the application must adhere to file name conventions as described in Annex 2 of the WHO IMD-PQS Guidelines for Prequalification.
Method to submit the pre-submission package
The applicant must submit the pre-submission, using the correct file name conventions, by email to Isaac Gobina (gobinai@who.int) and Paul Mallins (mallinsp@who.int) and Lauren Goodwin (goodwinl@who.int).
Individual PDF or Word files should not exceed 10 MB in size.
IMD-PQS response times: pre-submission process
The IMD-PQS Secretariat will render a decision on a complete pre-submission package within 30 days.