Prequalification application procedure
Prequalification application procedure: overview
An application dossier must include:
- complete details of the manufacturing process
- documented evidence that the product meets the performance and functional requirements detailed in the relevant performance specification
- complete manufacturing certification and licensing
WHO ePrequalification System
If the WHO IMD-PQS Secretariat deems the product or device eligible for prequalification evaluation, it will invite the manufacturer or reseller to submit a full prequalification application. As of July 2025, applications may ONLY be submitted via the WHO e-prequalification (ePQS) system, an online platform for prequalification applications.

ePQS Registration Form
To register on ePQS please complete the registration form: ePQS - User Registration and accessing the ePQS Portal and return by email to imd_amd@who.int, copying huckerbyg@who.int.
The ePQS landing page is accessible at: https://extranet.who.int/prequal/epqs-portal
Registration is rolled out on a need-basis: only the manufacturer or reseller of a new product application will be invited to register on ePQS during H1 2026. ePQS registration will be opened universally to all prequalification-holders in H2 2026.
ePQS Learning Materials & Troubleshooting
WHO IMD ePQS Learning Materials for PQ-holders & Applicants - provides step-by-step guidance for navigating the ePQS system and submitting applications
WHO IMD ePQS Frequently Asked Questions FAQs & Troubleshooting - *NEW for 2026* for IMD prequalification applicants
WHO ePQS General Troubleshooting - a general guide to common troubleshooting solutions for all ePQS users
WHO IMD-PQS Guidelines for Prequalification Holders - provides a brief outline and a technical introduction to ePQS in Annex 7
It is strongly recommended to read the guidance documents before submitting your application on ePQS.
Help with ePQS
In case technical assistance is required, please contact epqs@who.int, copying huckerbyg@who.int.
Application requirements (application dossier contents)
The IMD-PQS Secretariat will send the applicant an application information pack which contains:
- the complete application instructions and list of required documentation,
- the relevant Performance Specifications & Verification Protocols,
- an Application Review Template,
- a signed copy of the WHO IMD-PQS Terms & Conditions
- other related materials specific to each product and its prequalification.
A generic list of requirements for the application dossier contents is provided on the page: "Application Dossier Requirements".
Application Review Template
The IMD-PQS Application Review Template is a Microsoft Excel (.xls) document intended to collect and record and track information about the dossier completion status and the communication exchanges between the applicant and the IMD-PQS Secretariat that will take place throughout the dossier review process. Each Application Review Template corresponds to an IMD-PQS Performance Specification.
Available Application Review Templates are available on the page: "Application Dossier Requirements". If your application relates to a Performance Specification that does not yet have a corresponding Application Review Template, please contact the IMD Secretariat.
Important general information for prospective applicants
- One dossier must be submitted for EACH product.
- If the product is manufactured at more than one manufacturing site, one dossier must be submitted for EACH manufacturing site.
- All documents submitted as a part of the application must adhere to file name conventions as described in Annex 3 of the WHO IMD-PQS Guidelines for Prequalification Holders, and in the prequalification information pack.
- Dossier documents must be uploaded to ePQS using a MANDATORY folder structure. This folder structure is provided for download as a .zip folder here, and include detailed explanation and guidance on how to upload these documents to ePQS.
Method to submit the prequalification application dossier
Applicants must submit the pre-submission, using the correct file name conventions, by email to Dr Isaac Gobina (gobinai@who.int) and Paul Mallins (mallinsp@who.int), copying imd_amd@who.int.
Individual PDF or Word files should not exceed 10 MB in size.
New applications submitted as of June 2025 - Applicants must submit the application dossier exclusively via the WHO ePQS platform. Submissions by email or by postal mail will no longer be accepted. Applications on the ePQS platform may be created at any time, once the applicant has received an invitation to register and apply, and information pack from the IMD-PQS Secretariat.
To register on ePQS, please complete the form "ePQS - User Registration and accessing the ePQS Portal" and return by email to ime_amd@who.int (imd_amd@who.int), copying huckerbyg@who.int.
IMD-PQS prequalification fees administration
The dossier evaluation fees must be paid in full once the dossier has been accepted for evaluation. Payment should be made within 30 days of receipt of the invoice from WHO. The commencement of dossier evaluation will be triggered by a confirmation of payment of the invoiced fees.
Product testing
Laboratory testing
Laboratory testing is required for the majority of products submitted for IMD-PQS prequalification. For syringe categories E008 and E013, in-house testing by the product manufacturer is acceptable in lieu of testing by a WHO-accredited laboratory. Please refer to the "combined specification and testing protocol" standards for E008 and E013 for information regarding manufacturer testing certification requirements.
The type of laboratory testing required for all other products is defined in the relevant Verification Protocol(s), and is defined by the Secretariat based on the volumes that will be deployed and whether the product is safety-critical.
Laboratory testing results must be submitted to the IMD-PQS Secretariat using the IMD-PQS Laboratory Test Report Template.
The three types of testing are:
- Type-examination: An inspection of a production-run product. Required for items that are not programme-critical.
- Independent type-testing: An inspection and a rigorous test of production-run product. Required for programme-critical products.
- Full quality assurance: An inspection of the production site carried out against a pre-defined checklist. Required for complex programme-critical products involving site-specific design and on-site installation work.
All independent type-testing must be carried out by an accredited testing laboratory. Type-examination or full quality assurance can be carried out either by an accredited laboratory or by an independent specialist appointed by IMD-PQS.
A list of accredited laboratories is provided on the page "Accredited laboratories".
Field testing
In some cases, the results of additional testing of a product or device in its intended operating environment may be required, for inclusion in the application dossier. WHO is responsible for identifying product types for which field-testing is either mandatory or desirable and will specify the appropriate generic testing method for each product type.
Field-testing will always be required for:
- products that require the establishment of a new IMD-PQS category, and for
- products that are based on technologies that are new to the Expanded Programme on Immunization.
Complete information about field-testing for applicants is provided on the page: "Product testing support for manufacturers".
Field-testing also provides product manufacturers with information to improve product design, and can further help end-users to select products tat are best suited to their needs and operating environments.
Dossier screening
Each product dossier is screened for completeness before being evaluated, to make sure that all the required information and documentation have been submitted.
If the dossier is incomplete:
- the applicant will be contacted by email (and via the WHO ePQS platform as of Quarter 1 2025), and given a single opportunity to provide the missing information or production-run products within two-weeks.
If, after the two-week period has elapsed, the applicant fails to supply the missing information or production-run product, the dossier will be rejected.
Dossier evaluation
Once a product dossier has been accepted it will undergo review by a group of technical specialists. This group is convened twice a month to review application dossiers and laboratory test results, as well as share its recommendations as to whether products meet the relevant IMD-PQS equipment performance specifications. The IMD-PQS Secretariat and technical specialists treat all information pertaining to a dossier evaluation with the strictest confidence.
During dossier evaluation, the IMD-PQS Secretariat will inform applicants whether any clarifications or additional information is required before a final decision regarding prequalification can be taken.
If clarification or additional information is required of the applicant during the dossier evaluation phase:
- Applicants must ensure to respond with the complete information requested. The number of rounds of review for an application is strictly limited to three.
- In the case that the application is not approved after three rounds of review, the application will be rejected. If the applicant chooses to resubmit, the process begins again: the screening and dossier evaluation will recommence from the beginning, as will the review timelines. Anny new application must be accompanied by the payment of a new dossier review fee.
The dossier submission and evaluation process can be straightforward provided the dossier is complete, prepared as per the latest product specifications and all licences are up to date. If field evaluation and/or other validation of a product are required, results and/or laboratory tests outcomes must be included in the dossier.
Note: All applications relating to single-use injection devices will be processed strictly in accordance with the procedure described in WHO/BCT/03.09: Procedure for assessing the acceptability, in principle, of single-use injection devices for procurement by United Nations agencies.
Dossier evaluation timelines
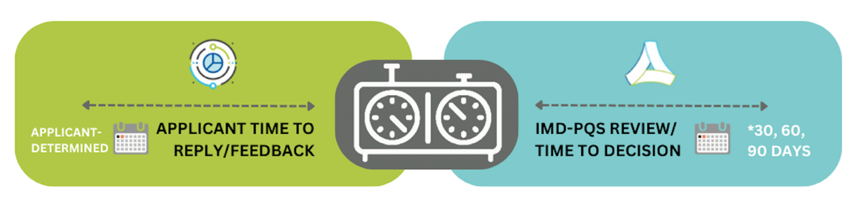
IMD-PQS will contact the manufacturer within 60 days of receiving the dossier depending on the number of clarification questions raised by the dossier review team (with the exception of category E001, for which IMD-PQS will respond within 90 days).
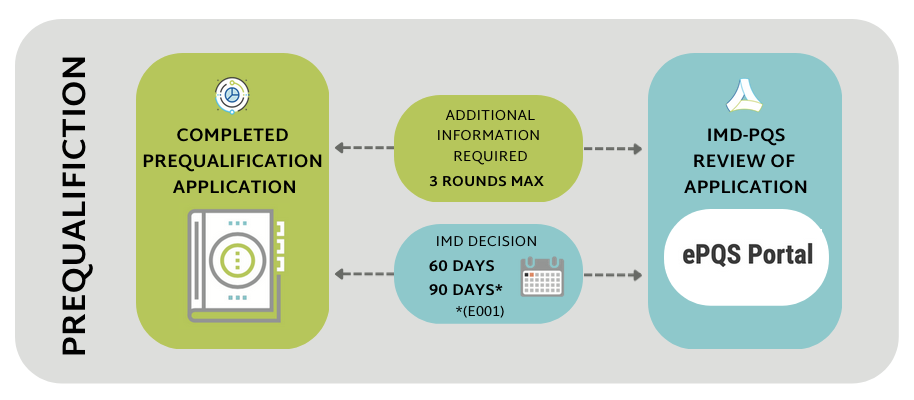
IMPORTANT: IMD-PQS total response time excludes wait-times whilst applicants are gathering and submitting the requested additional information.
Prequalification
Once a product has been approved for prequalification, IMD-PQS will inform the applicant of this decision via the WHO ePQS platform. Details of the approved product are then "published" on this website and in the WHO IMD-PQS Catalogue of Prequalified Products.
Post-prequalification
Following prequalification, a product must meet a number of post-prequalification commitments and obligations in order to maintain prequalified status. Failure to do so could result in withdrawal of prequalified status.
Prequalification guidelines
IMD-PQS Guidelines for Prequalification Holders - ALL PQS CATEGORIES
Prequalifiation checklists
E001 product dossier checklist
E003 product dossier checklist
E004 product dossier checklist
E006 product dossier checklist
E007 product dossier checklist
Presubmission form
Prequalification of immunization equipment and devices pre-submission form