E006: Temperature Monitoring Devices
The products in this category include thermometers, freeze indicators, temperature recorders, data loggers, Equipment Monitoring Devices (EMDs) and event loggers for monitoring temperatures and other variables at all levels in the cold chain, including alarm systems.
Details regarding the products included in each sub-category of E006, as well as guidelines, performance specifications and verification protocols for this category, can be accessed through the drop-down sections below.
E006 PRODUCT DATA SHEETS: Category E006 product data sheets are available for view, download and comparison here.
A number of different temperature monitoring device types are specified by PQS based on their particular applications in the cold chain. These range from more basic indicator and thermometers to advanced monitoring and communication systems. Employers of temperature monitoring should be familiar with the use-cases for the different types of devices and when best to deploy each type based on their specific programme needs and strategies.
E006.1.1: Alarms
Acoustic and visual alarms: These can be used in conjunction with fixed dial thermometers and pen recorders.
E006.1.2: Indicators
Cold Chain Monitor cards (CCM): WHO no longer recommends the use of these cards for in-country use. Their use should now be confined to international shipments only where dry ice is used. For more information and guidance on the use of CCMs, consult the WHO How to monitor temperature in the vaccine supply chain.
Freeze indicators: Freeze indicators should be used routinely for internal distribution of freeze-sensitive vaccines and for monitoring freezing events in cold rooms and vaccine refrigerators where alternative devices are not fitted, or are not thought to be reliable.
Vaccine vial monitors (VVM): VVMs or combined VVM/Threshold indicators are now routinely fixed to all vaccines supplied by UNICEF.
E006.1.3: Thermometers (no recording)
Electronic thermometers: Hand-held electronic thermometers are used by cold chain technicians during repair work, for routine monitoring, for cold chain studies and during the commissioning of cold rooms and freezer rooms.
Fixed dial thermometers: Fixed gas or vapor pressure dial thermometers require no power supply and they can be used to trigger an alarm system. Their primary use is as a back-up device for cold rooms and freezer rooms. Bi-metallic dial thermometers are no longer recommended because they easily lose their calibration.
Stem thermometers: A stem thermometer can be fitted in refrigerators, freezers, cold rooms and freezer rooms as an inexpensive back-up device for required 30-day temperature loggers. Stem thermometers should never be used as the primary temperature monitoring device because they do not provide a continuous record of vaccine temperature exposure. Bi-metallic dial thermometers are no longer recommended by WHO because they do not maintain their calibration.
Integrated electronic thermometer with alarm: This device type may be built-in to a vaccine refrigerator or freezer at the manufacturer’s discretion. Its purpose is to provide some of the capabilities of the monitoring equipment used on cold rooms and freezer rooms.
E006.1.4: Temperature recorders
Programmable remote temperature and event monitoring systems: Event monitoring systems consist of a network of sensors linked to a central temperature recording unit, which may connect and communicate with local and remote servers and services. Both hard-wired and wireless devices are available and devices of both types have integrated alarm systems. Detailed temperature records can be produced and the most sophisticated systems can be internet-enabled which allows for remote monitoring.
Pen recorders: Pen recorders have been standard equipment for cold rooms and freezer rooms for many years. They continue to have a use for smaller cold rooms and for programmes which are unable to provide the technical support needed to operate and maintain an event monitoring system.
Temperature data loggers: Data loggers are used principally during cold chain studies and for verifying performance during the initial commissioning of cold rooms and freezer rooms. Temperature data loggers have limited functionality. More advanced features can be found in 30-day electronic refrigerator temperature loggers.
30-day electronic refrigerator temperature loggers for vaccine refrigerators or freezers: All refrigerators must come equipped with an integrated Level 2 EMD, integrated Level 3 EMD or 30-day temperature logger device in compliance with WHO IMD-PQS standards. Refer to Table 2. This device type can be used to review vaccine refrigerator temperatures over 30 days. The earliest data points are continuously overwritten so that the user always has access to the most recent 30-day period. The devices include a visual temperature alarm and some models allow data to be downloaded to a computer. Their use offers the possibility of much improved temperature monitoring at the health facility and lower sub-national levels where routine manual recording is known to be unreliable.
Models acceptable for WHO prequalification must operate for a minimum of two years after activation. The whole unit has to be replaced when the battery runs out because the product is supplied with sealed in batteries. This design approach avoids the need to recalibrate the device, which is expensive and logistically complex.
Electronic shipping indicators: Electronic shipping indicators are single-use devices designed to monitor vaccine temperature during international shipment from the manufacturer to the primary store. The data they provide is recorded on the Vaccine Arrival Report (VAR).
E006.1.5: Equipment Monitoring Systems (EMS)
The Equipment Monitoring System (EMS) enables the monitoring of cold chain equipment. The EMS consists of three levels of functionality, performed by Equipment Monitoring Devices (EMDs). EMDs are the primary means of communicating monitored cold chain data locally and/remotely via the Internet. EMDs source raw data directly from the appliance's integrated logger. Vaccine refrigeration appliances are required to achieve Level 1 as minimum. Levels 2 and 3 can be integrated into the appliance or can be external to it. Refer to Table 2.
Level 1 is the datalogger with power and data connections for the Machine-to-machine interface (M2M). Note: more advanced local or remote means of communicating data re not required. The complete requirements of Level 1 are specified in IMD-PQS performance specification WHO/PQS/E006/DL01. The datalogger is primarily tasked with maintaining relative time, recording appliance data objects, generating and recording logger data objects and making that data available in a standardized way to other equipment monitoring devices and systems.
Level 2 is the Equipment Monitoring Device (EMD) and shares the same requirements as Level 1. The EMD is the primary means of communicating monitored cold chain data locally. The EMD draws raw data directly from the appliance's integrated logger. The EMD may be integrated into the appliance or may be external. The requirements of Level 2 are specified in IMD-PQS performance specification WHO/PQS/E006/EM01.
Level 3 shares the same requirements as Level 2, with the additional feature of remote communications (e.g. GSM / SIM-based, Wi-Fi® etc.). As Level 2, the EMD may be integrated into the appliance or may be external.The requirements of Level 3 are specified in IMD-PQS performance specification WHO/PQS/E006/EM01, with special attention to remote communication modalities.
The following table summarizes the use of each of the non-EMS E006 device types:
Table 1: Use of temperature monitoring devices
Note: The table shows the appropriate locations for each type of device. It does not imply that ALL the devices listed for a particular location should be used at the same time.

Table 2: Required temperature monitoring devices for vaccine refrigerators: Possible monitoring configurations
(Refer to specifications RF03 and RF05 for further information).
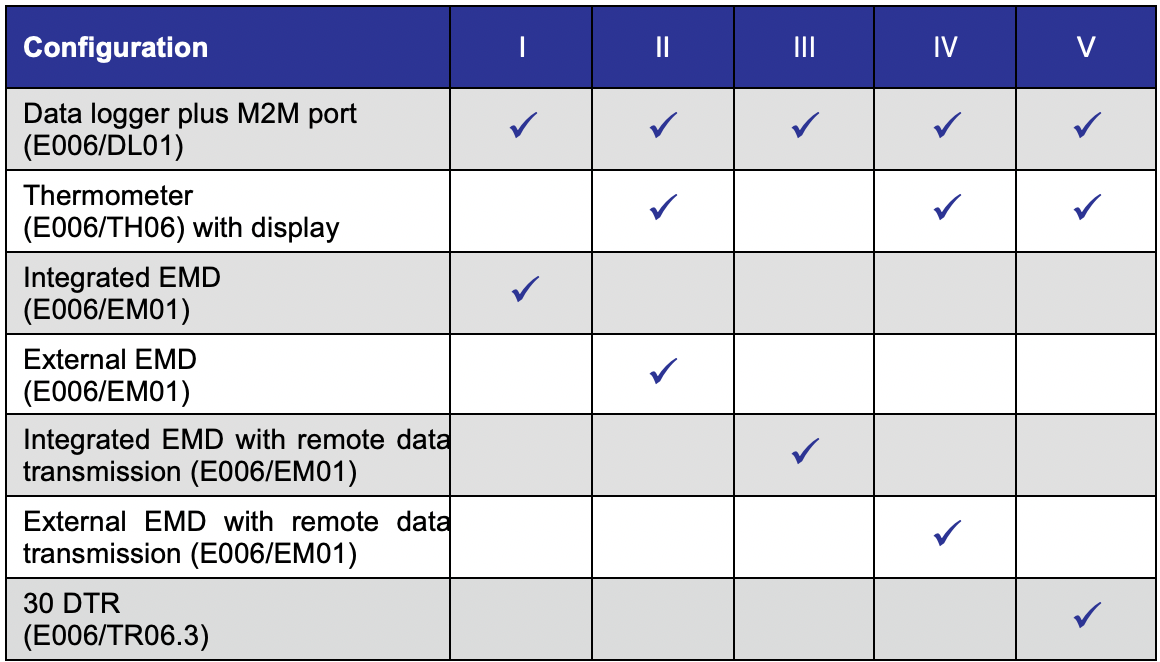
As of 2024, all NEW vaccine refrigerators are required to have an EMS. Starting in 2026, ALL vaccine refrigerators will be required to have an EMS. This new sub-category of temperature and equipment monitoring offers greater design flexibility and advanced functionality of cold chain monitoring. While temperature will still be the most essential CCE vaccine safety indicator for immunization programmes, there is increasing potential - and interest - in monitoring additional performance parameters of CCE, including voltage input, humidity, compressor function, etc. By monitoring and communicating additional parameters, EPI programmes, suppliers, service providers and global partners may gain deeper insights into the performance of CCE over time, enabling remote failure analysis and ideally predictive failure responses. This could ultimately lead to monitoring technology that helps programmes reduce CCE downtime and risk to vaccines.
E006.3.1: Solar power systems for low-energy requirements
Performance specifications for small, battery-based solar power systems intended for E006 devices such as RTMDs and other electronic monitpring systems (EMS) may also apply for low power requirements where only direct current electricity is required (e.g. 12 Vdc). See IMD-PQS performance specification WHO/PQS/E006/PVDC01: Solar power systems for low electrical requirements.
E006.3.2: Basic USB Energy Harvesting Controller (EHC)
Many solar appliances now have a Basic USB Energy Harvesting Controller (EHC). The Basic USB EHC provides up to 1A @ 5VDC (5W) on a USB-A port that may be used to recharge RTMDs and other monitoring devices. See IMD-PQS performance specification WHO/PQS/E007/EHC02.
PQS Equipment Monitoring Systems (EMS) Introduction
PQS Equipment Monitoring Systems (EMS) Frequently Asked Questions
Additional EMS Testing Requirements for DL01 devices
EMS Performance specifications
PQS Logger example root directory DL01.2 JSON (--> Document downloads as .zip to your local drive; check your downloads folder)
PQS performance specification E006/DS01.2: Data standards for Equipment Monitoring Systems
PQS performance specification E006/DS01.2: Annex 1 Cold Chain Data Objects
PQS performance specification E006/DS01.2 Annex 2 JSON Schema (--> Document downloads as .zip to your local drive, check your downloads folder)
EMS Verification protocols
PQS verification protocol E006/DS01-VP.2 Annex 3 JSON Verification Schema (--> Document downloads as .zip to your local drive, check your downloads folder)
Performance specifications
PQS performance specification E006/AL01.1: Acoustic and/or visual alarm units
PQS performance specification E006/IN02.1: Cold chain monitor
PQS performance specification E006/IN03.1: Irreversible freeze indicator
PQS performance specification E006/IN04.1: Threshold indicators
PQS performance specification E006/IN05.4: Vaccine vial monitor
PQS performance specification E006/IN06.1: Combined vaccine vial monitor and threshold indicator
PQS performance specification E006/IN07.1: Chemical freeze indicator
PQS performance specification E006/PVDC01: Solar power system for low electrical requirements
PQS performance specification E006/TH01.1: Portable electronic thermometer
PQS performance specification E006/TH02.2: Fixed gas or vapour pressure dial thermometer
PQS performance specification E006/TH03.1: Portable alcohol stem thermometer
PQS performance specification E006/TR04.1: Wall-mounted pen recording thermometer
PQS performance specification E006/TR05.1: User-programmable temperature data loggers
PQS performance specification E006/TR06.4: 30-day electronic refrigerator temperature logger
PQS performance specification E006/TR07.4: Electronic shipping indicators
Verification protocols
PQS type-examination protocol E006/AL01.VP-1: Acoustic and/or visual alarm units
PQS type-examination protocol E006/IN02.VP.1: Cold chain monitor
PQS independent type-testing protocol E006/IN03-VP.1: Irreversible freeze indicator
PQS independent type-testing protocol E006/IN04-VP.1: Threshold indicator
PQS independent type-testing protocol E006/IN05-VP.4: Vaccine vial monitor
PQS independent type-testing protocol E006/IN07-VP.1: Chemical freeze indicator
PQS type-examination protocol PVDC-VP1.0: Solar power system for low electrical requirements
PQS Type-examination protocol E006/TH01.VP.1 Portable electronic thermometer
PQS independent type-testing protocol E006/TH02-VP.2: Fixed gas or vapour pressure dial thermometer
PQS independent type-testing protocol E006/TH03-VP.1: Portable alcohol stem thermometer
PQS independent type-testing protocol E006/TR04-VP.1: Wall-mounted pen recording thermometer
PQS Type-examination protocol E006/TR05.VP-1 User-programmable temperature data loggers
PQS Independent type-testing protocol E006/TR07-VP.4 Electronic shipping indicators
Guidance for manufacturers or suppliers
IMD-PQS Guidelines for Prequalification Holders