Evaluation for Prequalification
Product evaluation and re-evaluation
Production evaluation for prequalification and the maintenance of prequalified status has five stages:
- Stage 1: Product application pre-submission
- Stage 2: Product prequalification application
- Stage 3: Application review and evaluation
- Stage 4: Prequalification
- Stage 5: Post-prequalification re-evaluation and commitments
Laboratory testing is required for the majority of products submitted for IMD-PQS prequalification. The type of laboratory testing required for each product is defined in the relevant verification protocol(s).
Prequalification Holders of IMD-PQS prequalified products (manufacturers and licenced resellers) commit to fulfill three types of post-prequalification commitments:
- the Annual Review of all prequalified products
- Post-market monitoring and reporting of product performance, and
- (if required) extra-ordinary product review.
Detailed information about these procedures can be found under Post-prequalification committments.
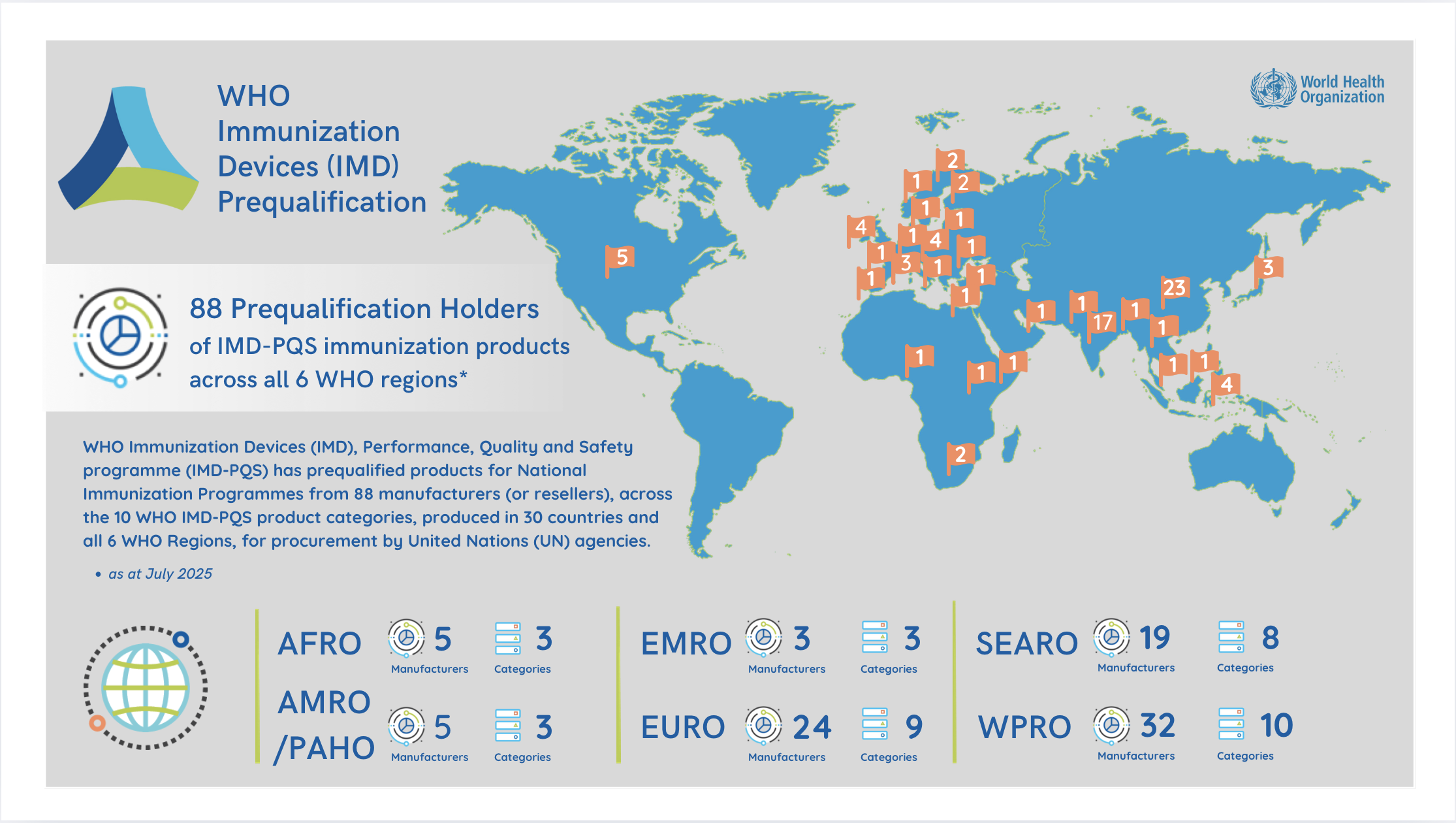
The value of immunization device prequalification
Rigorous evaluation and re-evaluation of prequalified products assures UN and other procurement agencies and end-users that products meet, and continue to meet robust standards of performance, quality and safety. These activities also ensure that Industry and Prequalification Holders have a fair basis for tendering, and that the evaluation process is impartial and reliable. Ultimately, the availability of prequalified products helps ensure that WHO immunization programmes are equipped with appropriate, quality and reliable equipment, thereby optimizing the reach, safety and impact of those programmes.