Assessment of Laboratories for Accreditation
WHO accredits laboratories that conform to international standards and codes of practice, and that can demonstrate to a competent third-party accreditation body that they meet these criteria. Laboratories may be accredited to test one or several categories of products. They must also be able to carry out one or more of the three types of product verification test:
- Type-examination: An inspection of a production-run product. Required for items that are not programme-critical.
- Independent type-testing: An inspection of the manufacturing site and a rigorous test of production-run product. Required for products that are programme-critical.
- Full quality assurance: An inspection of the production site carried out against a pre-defined checklist. Used for complex programme-critical products involving site-specific design and on-site installation work.
All independent type-testing must be carried out by an accredited testing laboratory. In addition, accredited testing laboratories may sometimes be used to carry out type-examination or full quality assurance of a product.
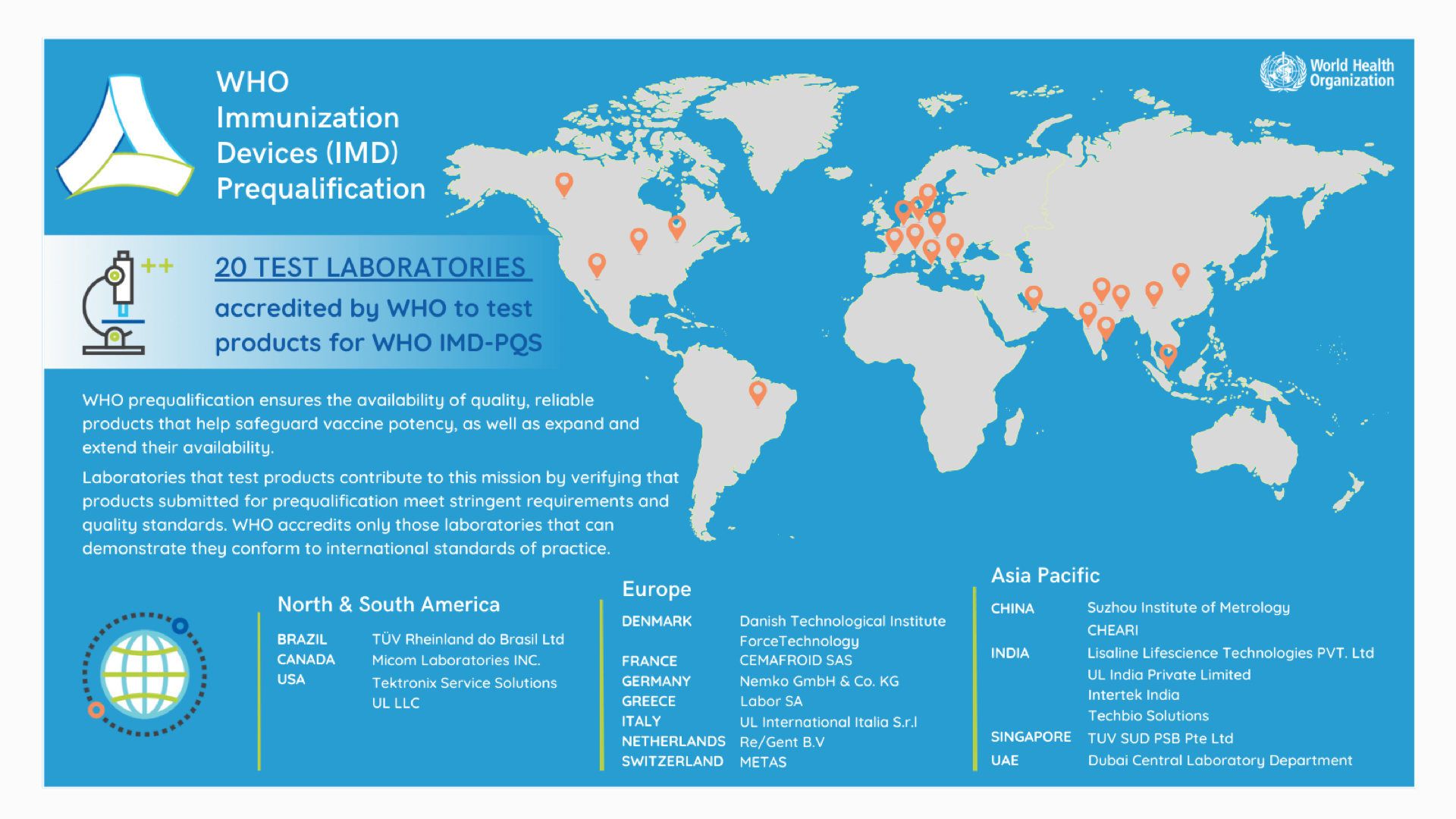
Accredited laboratories are currently located across Europe, North and South America, Asia Pacific and the Middle East.
Only laboratories accredited by WHO can be selected to test immunization products for WHO prequalification. WHO is currently seeking new laboratories that meet WHO accreditation criteria.
Assessment principles
WHO assessment of laboratories is guided by the following principles:
- Internationally-recognized standards: All laboratories must demonstrate a robust quality management system and compliance with ISO/IEC 17025 General requirements for the competence of testing and calibration laboratories.
- Roles and responsibilities: Roles and responsibilities of all personnel involved in carrying out the audit and assessment are clearly defined so that expectations can be met and the accountability of each is understood.
- Competence of the inspection team: Inspectors are selected based on auditing skills, education and experience in the regulatory requirements that are appropriate for the inspections and audit tasks assigned to them.
- Consistency: Assessment and audit procedures are performed according to defined guidelines, so that WHO can ensure consistency during the inspection. Guidelines are codified in the publicly available WHO standard operating procedures: How to assess a laboratory for PQS accreditation and How to re-evaluate a WHO PQS-accredited test laboratory.
- Confidentiality and standard of conduct: Auditors maintain confidentiality with regard to information and documentation related to inspections and must comply with WHO standards of conduct.
Benefits of WHO laboratory audits and accreditation
WHO audit and accreditation procedures help to build the capacity and availability of product-testing laboratories that can verify products' full compliance with the relevant performance specifications and verification protocols, using stringent examination, testing and quality.
WHO guidance
SOP 007: How to assess a laboratory for PQS accreditation
SOP 008: How to re-evaluate a WHO PQS-accredited test laboratory
International Organization for Standardization/International Electrotechnical Commission guidance
ISO/IEC 17025 General requirements for the competence of testing and calibration laboratories