SF - Immunization Devices
Download file
Product Details
Product name
Yushou®
IMD-PQS Code
E013-136
Status
Prequalified
Date of acceptance
15 Jan 2024
Appliance type
E013
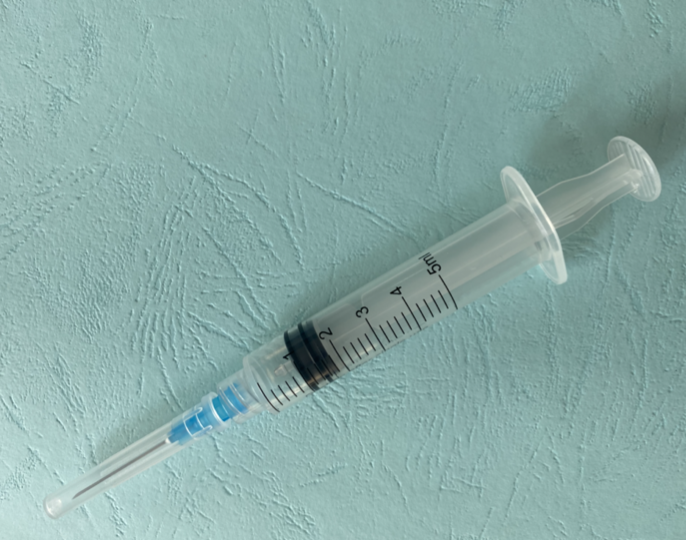
Product description
RUP syringe 5ml
Manufacturer
Wuxi Yushou Medical Appliances Co., Ltd
Manufacturer Reference
Yushou®
Country of Manufacture
China
Address
No.115 Nongxinhe Road Xishan District
Wuxi city
Jiangsu
China
Wuxi city
Jiangsu
China
Telephone
+86 (510)3777555
Email
Website address
Valid until
31 May 2026
Specifications
Product specifications - Main
Vaccine capacity (mL)
5.00
Graduations (ml)
0.20
Needle Size
23Gx1" (0.60 x 25 mm)
Needle Fixation
Detached
Syringe material(s)
Polypropylene
Number of pieces
2000.00
Location RUP activation
End of injection
RUP ISO Type
Type 1A
RUP Primary Packaging
Paper blister pack
RUP Sub-category
Simple RUP
Price and shipping
Shipping volume (m3)
0.135
Shipping weight (kg)
15.00
Units per package
3.00
Pieces per package
2000
Minimum order
200000.00
Price 1
POA
Year of base price
2023
Incoterms
FOB
Quality standard
Quality Standard
ISO 13485
Specification Reference
Applicable standard for specification & verification: ISO 7886-4
Product Sites
Wuxi Yushou Medical Appliances Co., Ltd
No.115 Nongxinhe Road Xishan District
Wuxi city,
Jiangsu
China
Document Details