SF - Immunization Devices
Download file
Product Details
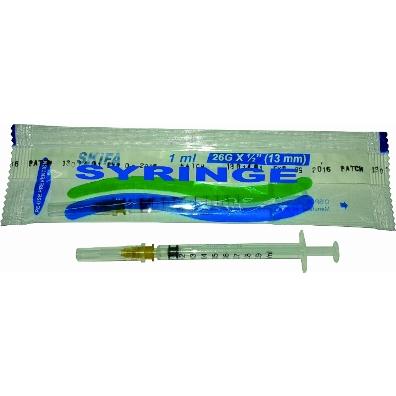
Product name
SKIFA RUP 1ml
IMD-PQS Code
E013-110
Status
Prequalified
Date of acceptance
05 Jul 2015
Appliance type
E013
Product description
RUP syringe 1ml
Manufacturer
Mitra Rajawali Banjaran, PT
Manufacturer Reference
SKIFA RUP 1ml
Address
JL. Raya Banjaran KM16
Bandung
Jawa Barat
Indonesia
Bandung
Jawa Barat
Indonesia
Telephone
+62 22 594 0151
Email
Website address
Valid until
31 May 2025
Specifications
Product specifications - Main
Intended purpose
Medication variable dose administration;Medication variable dose reconstitution
Restrictive use for
None
Vaccine capacity (mL)
1.00
Graduations (ml)
0.05
Needle Size
26Gx3/8" (0.45 x 10 mm)
Needle Fixation
Detached
Nominal capacity (ml)
1.00
Packaging & Sterility Assurance
Yes
Plunger aspiration
Yes
Number of pieces
3.00
RUP mechanism
Hooked to hub at end of injection
Location RUP activation
End of injection
RUP Nominal Capacity (ml)
1.00
RUP plunger Aspiration
Multiple
RUP Primary Packaging
Blister pack
RUP Sub-category
Simple RUP
Price and shipping
Shipping volume (m3)
0.142
Shipping weight (kg)
11.88
Pieces per package
100
Minimum order
10000.00
Price 1
24cts
Year of base price
2015
Incoterms
EXW
Quality standard
Quality Standard
ISO 13485
Specification Reference
Applicable PQS specification: ISO 7886-4
Applicable PQS VP(s): ISO 7886-4
Applicable PQS VP(s): ISO 7886-4
Product Sites
Mitra Rajawali Banjaran, PT
JL. Raya Banjaran KM16
Bandung,
Jawa Barat
Indonesia
Document Details