SF - Immunization Devices
Download file
Product Details
Product name
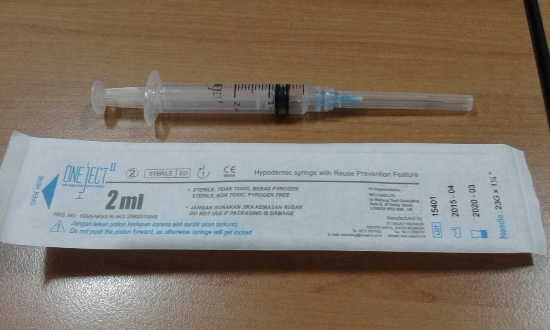
Oneject II RUP Syringe 2 mL
IMD-PQS Code
E013-108
Status
Prequalified
Date of acceptance
21 Sep 2015
Appliance type
E013
Product description
RUP syringe 2 ml
Manufacturer
PT Oneject Indonesia
Manufacturer Reference
Oneject II RUP Syringe 2 mL
Address
JL. Anggrek VII No.41, Kawasan lndustri Terpadu Indonesia China (KITIC),
Kab. Bekasi
Jawa Barat
Indonesia
Kab. Bekasi
Jawa Barat
Indonesia
Telephone
+62 21 879 17 422
Website address
Valid until
31 May 2026
Specifications
Product specifications - Main
Intended purpose
Medication fixed dose administration
Restrictive use for
Therapeutic use
Graduations (ml)
0.10
Needle Size
Other
Needle Size (Other)
23G x 1 1/4" (30 mm)
Needle Fixation
Fixed
Nominal capacity (ml)
2.00
Packaging & Sterility Assurance
Yes
Plunger aspiration
Yes
Number of pieces
3.00
RUP mechanism
Locked / broken plunger
Location RUP activation
End of injection
RUP Nominal Capacity (ml)
2.00
RUP plunger Aspiration
Multiple
RUP Primary Packaging
Blister pack
RUP Sub-category
Simple RUP
Price and shipping
Shipping volume (m3)
0.09
Shipping weight (kg)
10.60
Pieces per package
1600
Minimum order
1600.00
Price 1
Upon request to manufacturer
Year of base price
2015
Incoterms
FOB
Quality standard
Quality Standard
ISO 13485;Other
Quality standard (Other)
ISO 9001
Specification Reference
Applicable PQS specification: ISO 7886-4
Applicable PQS VP(s): ISO 7886-4
Applicable PQS VP(s): ISO 7886-4
Product Sites
PT Oneject Indonesia
JI. Anggrek VII No.41, Kawasan lndustri Terpadu Indonesia China (KITIC),
Kab. Bekasi,
Jawa Barat
17337
Indonesia
Document Details