SF - Immunization Devices
Download file
Product Details
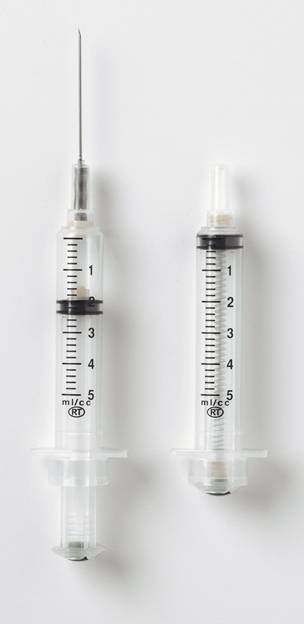
Product name
VanishPoint® Retractable 5ml
IMD-PQS Code
E013-008
Status
Prequalified
Date of acceptance
16 Jun 2005
Appliance type
E013
Product description
RUP retractable syringe 5ml
Manufacturer
Retractable Technologies, Inc.
Manufacturer Reference
VanishPoint® Retractable 5ml
Country of Manufacture
China
Address
511 Lobo Lane,
Little Elm
Texas
United States of America
Little Elm
Texas
United States of America
Telephone
+1 972 294 1010
Email
Website address
Valid until
31 May 2026
Specifications
Product specifications - Main
Intended purpose
Medication variable dose administration
Restrictive use for
None
Vaccine capacity (mL)
5.00
Graduations (ml)
0.20
Needle Size
Other
Needle Size (Other)
21G x 1 1/2" (38 mm)
Needle Fixation
Fixed
Nominal capacity (ml)
5.00
Packaging & Sterility Assurance
Yes
Plunger aspiration
Yes
Number of pieces
3.00
RUP mechanism
Retraction of needle into barrel by spring
Location RUP activation
End of injection
RUP Nominal Capacity (ml)
5.00
RUP plunger Aspiration
Multiple
RUP Primary Packaging
Paper blister pack
RUP Sub-category
Retractable RUP
Comments
The retraction of the needle is a reuse prevention feature (RUP) and a Sharp Injury Protection (SIP)
Price and shipping
Shipping volume (m3)
0.045
Shipping weight (kg)
8.20
Pieces per package
600
Minimum order
600.00
Price 1
On request
Year of base price
2005
Incoterms
FCA
Quality standard
Quality Standard
ISO 13485
Specification Reference
Applicable PQS specification: ISO 7886-4
Applicable PQS VP(s): ISO 7886-4
Applicable PQS VP(s): ISO 7886-4
Product Sites
Jumin Bio-Technologies Co., Ltd
396 Renjie South Road Fengxian District
Shanghai,
Shanghai
201400
China
Document Details