SF - Immunization Devices
Download file
Product Details
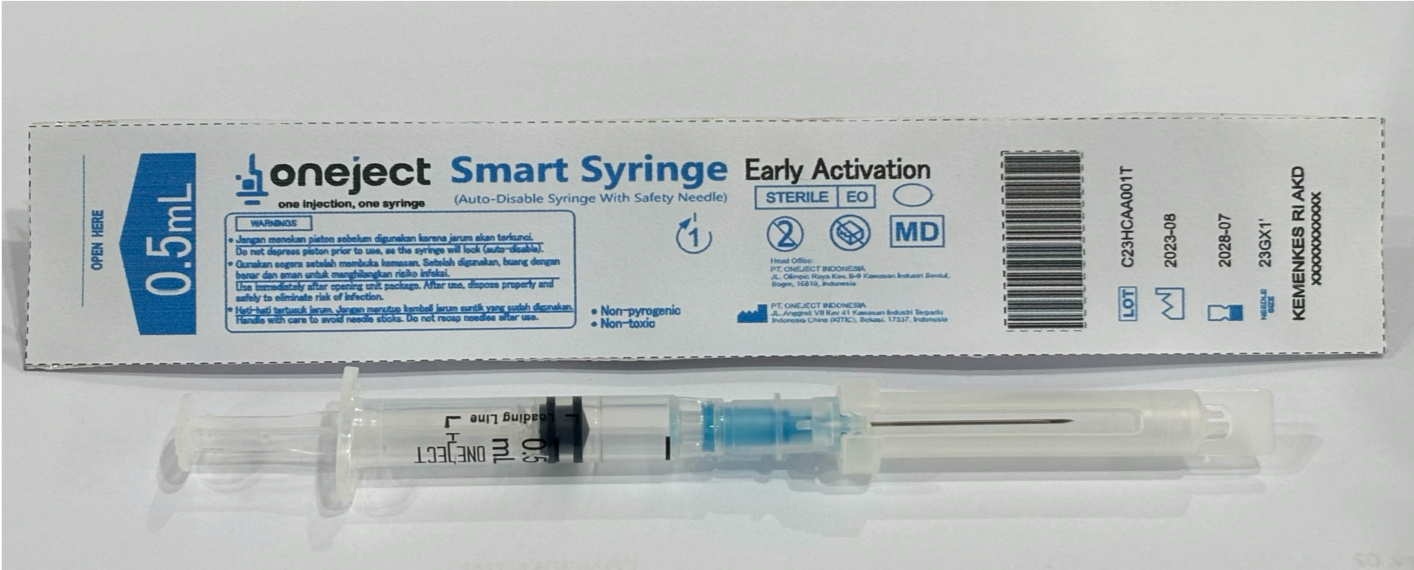
Product name
Oneject 0.5 AD Syringe with SIP Feature
IMD-PQS Code
E008-112
Status
Prequalified
Date of acceptance
07 May 2025
Appliance type
E008
Product description
AD syringe 0.5ml
Manufacturer
PT Oneject Indonesia
Manufacturer Reference
Oneject 0.5 AD Syringe with SIP Feature
Country of Manufacture
Indonesia
Address
JL. Anggrek VII No.41, Kawasan lndustri Terpadu Indonesia China (KITIC),
Kab. Bekasi
Jawa Barat
Indonesia
Kab. Bekasi
Jawa Barat
Indonesia
Telephone
+62 21 879 17 422
Website address
Valid until
31 May 2026
Specifications
Product specifications - Main
Vaccine capacity (mL)
0.50
Graduations (ml)
0.50
Syringe material(s)
Polypropylene
RUP mechanism
Locked / Broken plunger
Units per package
100.00
AD Number pieces
3
Auto-disabling at:
Start of injection
Needle Size
23Gx1" (0.60 x 25 mm)
AD Primary packaging
Blister pack
Price and shipping
Shipping volume (m3)
0.0048
Weight per Unit (Kg)
0.6420
Minimum order (unit)
1000.00
Price 1
Upon request to manufacturer
Year of base price
2025
Incoterms
FOB
Quality standard
Quality Standard
ISO 13485;Other
Quality standard (Other)
MDSAP
Specification Reference
1. EN ISO 13485 : 2016 (MD 760987)
2. US 21 CFR part 820
3. GMP-MD Indonesia by Ministry of Health
2. US 21 CFR part 820
3. GMP-MD Indonesia by Ministry of Health
Product Sites
PT Oneject Indonesia
JI. Anggrek VII No.41, Kawasan lndustri Terpadu Indonesia China (KITIC),
Kab. Bekasi,
Jawa Barat
17337
Indonesia
Document Details