SF - Immunization Devices
Download file
Product Details
Product name
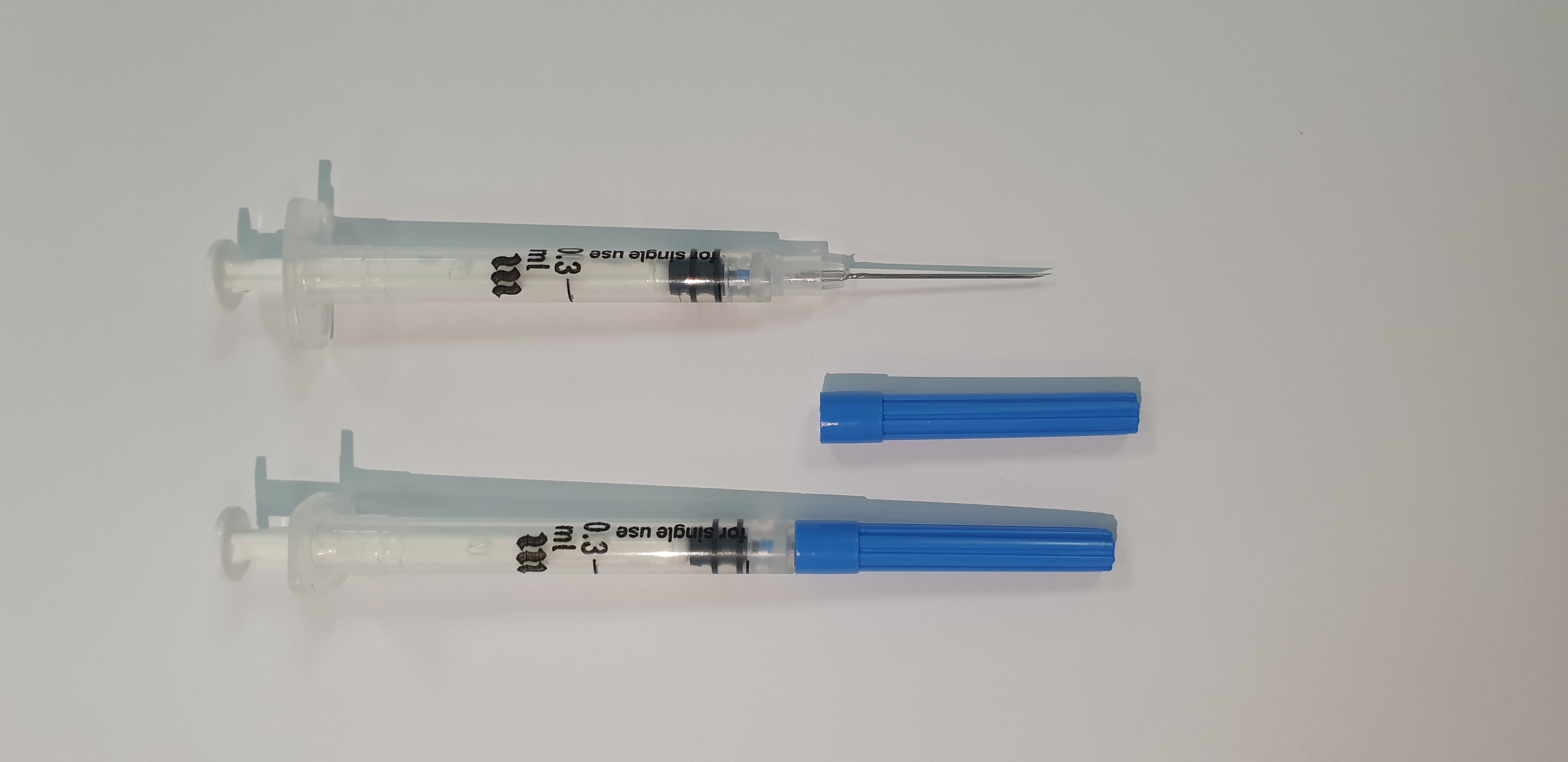
MEDECO INJECT AD 0.3ml
IMD-PQS Code
E008-081
Status
Prequalified
Date of acceptance
13 Jan 2021
Appliance type
E008
Product description
AD syringe 0.3ml
Manufacturer
Abu Dhabi Medical Devices Co. L.L.C
Manufacturer Reference
MEDECO INJECT AD 0.3ml
Country of Manufacture
United Arab Emirates
Address
MUSSAFAH INDUSTRIAL AREA, M-43, PLOT 124
ABU DHABI
United Arab Emirates
ABU DHABI
United Arab Emirates
Telephone
+971-2-6199100
Email
Website address
Valid until
31 May 2026
Specifications
Product specifications - Main
Vaccine capacity (mL)
0.30
Markings
CE mark
Syringe material(s)
Polypropylene
RUP mechanism
Valve in the syringe hub
Packaging & Sterility Assurance
Yes
Units per package
3000.00
AD Number pieces
3 pieces
Auto-disabling at:
Start of injection
Needle Size
23Gx1" (0.60 x 25 mm)
AD Primary packaging
Blister pack
Comments
Graduation 0.3ml. Average deadspace 0.0231mL
Price and shipping
Shipping volume (m3)
0.1
Weight per package (Kg)
11.5000
Pieces per package
3000
Minimum order (unit)
100000.00
Price 1
0.054 USD
Year of base price
2021
Incoterms
EXW
Quality standard
Quality Standard
ISO 13485
Specification Reference
Applicable PQS specification: 7883-3
Applicable PQS VP(s): 78863-3
Applicable PQS VP(s): 78863-3
Product Sites
Abu Dhabi Medical Devices Co. L.L.C
MUSSAFAH INDUSTRIAL AREA, M-43, PLOT 124
ABU DHABI,
30485
United Arab Emirates
Document Details