SF - Immunization Devices
Download file
Product Details
Product name
Haiou 0.5ml Auto Disable Syringe
IMD-PQS Code
E008-076
Status
Prequalified
Date of acceptance
11 Apr 2019
Appliance type
E008
Product description
AD syringe 0.5ml
Manufacturer
Guangdong Haiou Medical Apparatus Co., Ltd
Manufacturer Reference
Haiou 0.5ml Auto Disable Syringe
Country of Manufacture
China
Address
Nanyuan Industrial Zone, North Liusha,
Puning City
Guangdong
China
Puning City
Guangdong
China
Telephone
+86-663-2758666
Email
Website address
Valid until
31 May 2026
Specifications
Product specifications - Main
Vaccine capacity (mL)
0.50
Graduations (ml)
0.50
Markings
CE mark
Syringe material(s)
Polypropylene
RUP mechanism
Plunger break
Packaging & Sterility Assurance
Yes
AD Number pieces
3 pieces
Auto-disabling at:
End of injection
Needle Size
23Gx1" (0.60 x 25 mm)
AD Primary packaging
Blister pack
Price and shipping
Shipping volume (m3)
0.086
Weight per package (Kg)
13.0000
Pieces per package
3000
Minimum order (unit)
100000.00
Price 1
on request
Price 2
on request
Year of base price
2019
Incoterms
FOB
Quality standard
Quality Standard
ISO 13485
Specification Reference
Applicable PQS specification: ISO 7886-3
Applicable PQS VP(s):
Applicable PQS VP(s):
Product Sites
Guangdong Haiou Medical Apparatus Co., Ltd
Nanyuan Industrial Zone, North Liusha,
Puning City,
Guangdong
China
Document Details
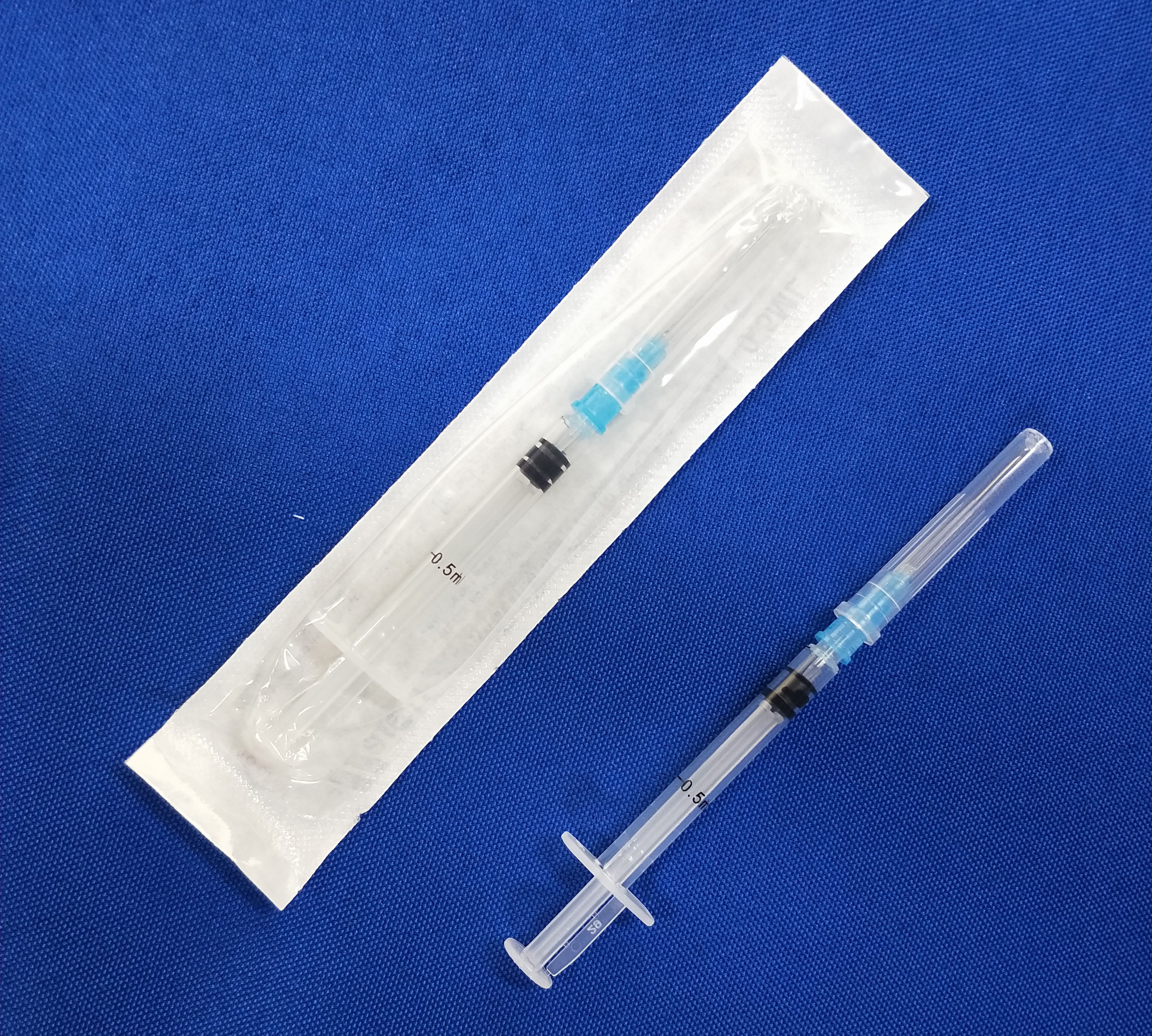
Product standard: ISO 7886-3