SF - Immunization Devices
Download file
Product Details
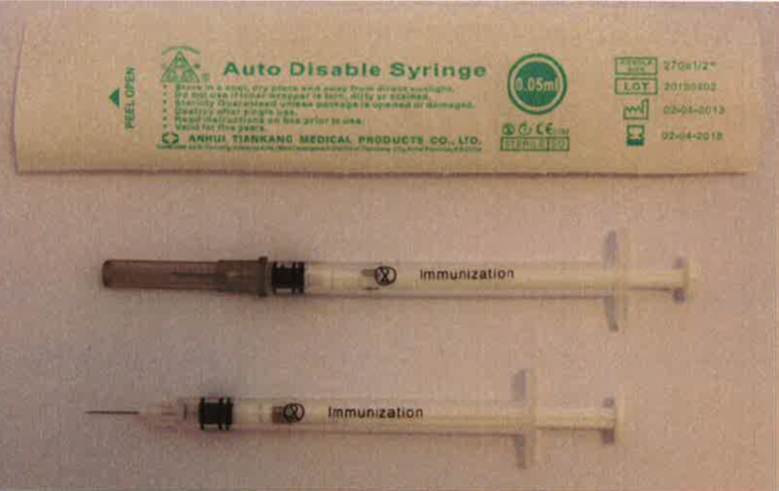
Product name
Auto-disable syringe 0.25ml
IMD-PQS Code
E008-058
Status
Prequalified
Date of acceptance
29 Jul 2013
Appliance type
E008
Product description
AD syringe 0.25ml
Manufacturer
Anhui Tiankang Medical Technology Co., Ltd
Manufacturer Reference
Auto-disable syringe 0.25ml
Country of Manufacture
China
Address
No. 228, Weiyi Road Economic Development Zone
Tianchang
Anhui
China
Tianchang
Anhui
China
Telephone
+86 550 7308592
Email
Website address
Valid until
31 May 2026
Specifications
Product specifications - Main
Vaccine capacity (mL)
0.25
Graduations (ml)
0.25
Markings
CE mark
RUP mechanism
Plunger lock by stainless steel jaw at start of injection
Packaging & Sterility Assurance
Yes
Units per package
100.00
AD Number pieces
3 pieces
Auto-disabling at:
Start of injection
Needle Size
27Gx3/8" (0.40 x 10 mm)
AD Sub Category
Simple AD
AD Primary packaging
Blister pack
Price and shipping
Shipping volume (m3)
0.069
Weight per package (Kg)
10.0000
Pieces per package
2000
Minimum order (unit)
200000.00
Price 1
Upon request
Year of base price
2013
Incoterms
FCA
Quality standard
Quality Standard
ISO 13485
Specification Reference
Applicable PQS specification: ISO 7886-3
Applicable PQS VP(s): ISO 7886-3
Applicable PQS VP(s): ISO 7886-3
Product Sites
Anhui Tiankang Medical Technology Co., Ltd
No 51 4th Group Wanjie Village Baodun Town Xinjin District
Chengdu City,
Sichuan
611438
China
Document Details