SF - Immunization Devices
Download file
Product Details
Product name
Auto-disable syringe 0.5ml BD SoloShot™ Mini
IMD-PQS Code
E008-035
Status
Prequalified
Date of acceptance
04 Aug 2009
Appliance type
E008
Product description
AD syringe 0.5ml
Manufacturer
Becton Dickinson
Manufacturer Reference
Auto-disable syringe 0.5ml BD SoloShot™ Mini
Country of Manufacture
Spain
Address
Crtra. Mequinenza (N-211) S/N
Fraga
Huesca
Spain
Fraga
Huesca
Spain
Telephone
+34 974470900
Email
Website address
Valid until
31 May 2026
Specifications
Product specifications - Main
Vaccine capacity (mL)
0.50
Graduations (ml)
0.50
Markings
CE mark
RUP mechanism
locking clip; locked trapped piston
Packaging & Sterility Assurance
Yes
Units per package
100.00
AD Number pieces
2 pieces
Auto-disabling at:
Start of injection
Needle Size
23Gx1" (0.60 x 25 mm)
AD Sub Category
Simple AD
AD Primary packaging
Blister pack
Price and shipping
Shipping volume (m3)
0.102
Pieces per package
3300
Price 1
On request
Year of base price
2009
Incoterms
FCA
Quality standard
Quality Standard
ISO 13485;Other
Quality standard (Other)
ISO 9001, ISO 7886-3
Specification Reference
Applicable PQS specification: ISO 7886-3
Applicable PQS VP(s): ISO 7886-3
Applicable PQS VP(s): ISO 7886-3
Product Sites
Becton Dickinson
Crtra. Mequinenza (N-211) S/N,
Fraga,
Huesca
22520
Spain
Document Details
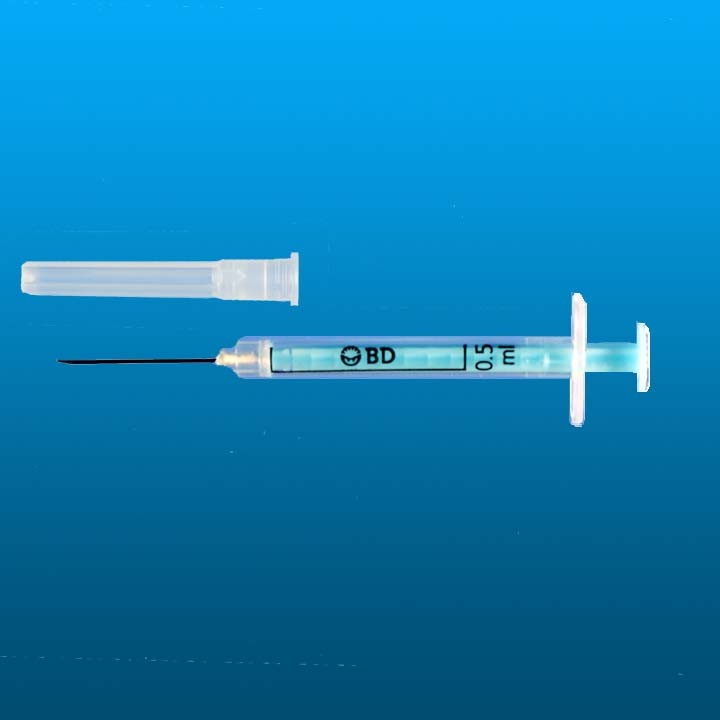
Minimum order (units): 3300 / 3600 /4320
Weight per package (kg): 10.45 / 10.20 kg