What We Do
The WHO Immunization Devices, Performance, Quality and Safety initiative (WHO IMD-PQS) is the group within the WHO prequalification that prequalifies products used to transport, store, monitor, administer and safely dispose of vaccines. WHO prequalification enables Member States and UN procurement agencies to be assured that products have been evaluated and deemed suitable and reliable for immunization programmes.
IMD-PQS contributes to the broad access agenda of WHO which seeks to strengthen regulation of health products and expand access to quality-assured products in countries that have a heavy disease burden, including vaccine-preventable diseases.
IMD-PQS also supports the Immunization Agenda 2030 (IA2030) priorities, including coverage and equity and supply and sustainability.
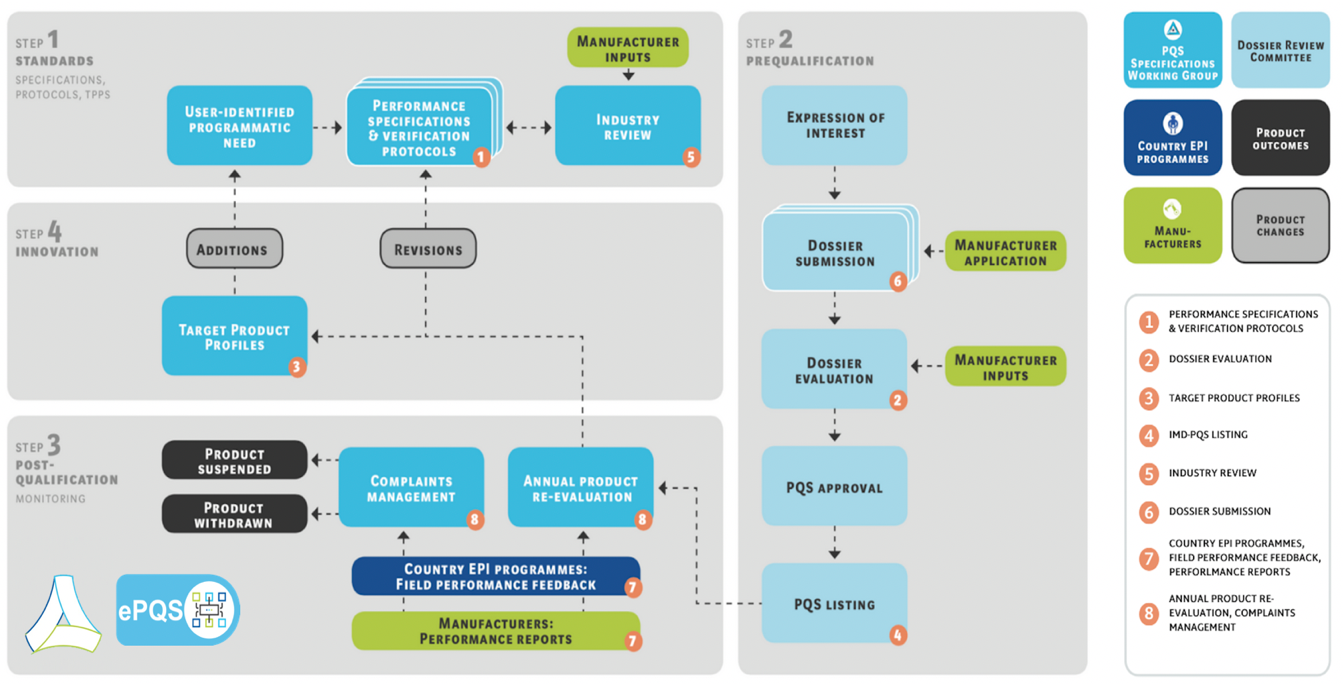
IMD-PQS works to ensure that: WHO Member States' national immunization programmes have access to products that have the requisite characteristics; that UN and other procurement agencies can procure products that are fit for purpose; and that prospective Prequalification Holders (manufacturers) have a fair basis for tendering products and investing in the development of future innovation. It does this by:
- setting standards and norms in the form of product specifications that define the performance, quality and the safety characteristics required for specific operating environments
- defining the product testing and verification protocols for determining whether a product meets the specified criteria
- evaluating whether products meet these performance specifications and are eligible for prequalification for use in immunization programmes
- reviewing product performance to determine whether the prequalified status of a product or device can be maintained or should be withdrawn
- assessing laboratories for accreditation to test immunization products undergoing evaluation for prequalification
- collecting product performance feedback from WHO Member States and Prequalification Holders, and gathering information from product end-users about their evolving needs to help stimulate innovation.
WHO Immunization Devices works to develop standards that will lead to the development of immunization cold chain products that mean countries' diverse and evolving needs:
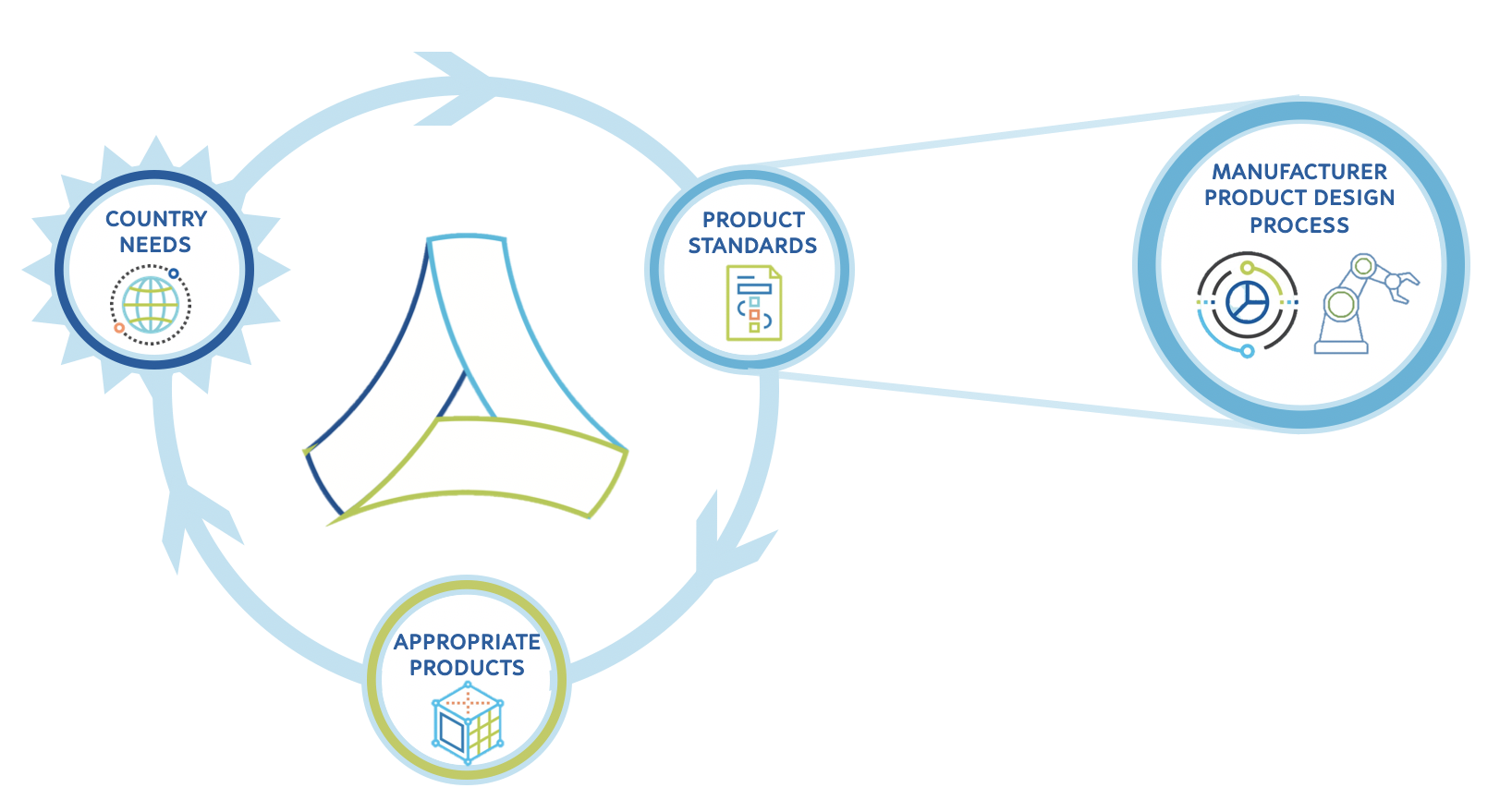
Principle outputs & publications of WHO Immunization Devices
- Catalogue of WHO-prequalified Immunization Devices
- performance specifications for immunization products
- product verification protocols for the testing and evaluation of products
- target product profiles that define the desirable characteristics for future innovations
- the WHO list of laboratories that are accredited to test for product compliance with prequalification standards
- guidance for prequalification applicants and research organizations carrying out field evaluations.
Explore WHO Immunization Device's mandate, strategy and process further:
Isaac Gobina, IMD-PQS Technical Officer, presents the mandate and impact at the 17th TechNet-21 conference (2023) in this video.
WHO PQS First line of defense for the immunization supply chain (17th TechNet Conference) - ENGLISH
PQS DE L'OMS : Première ligne de défense de la chaîne d'approvisionnement en vaccins - FRANCAIS

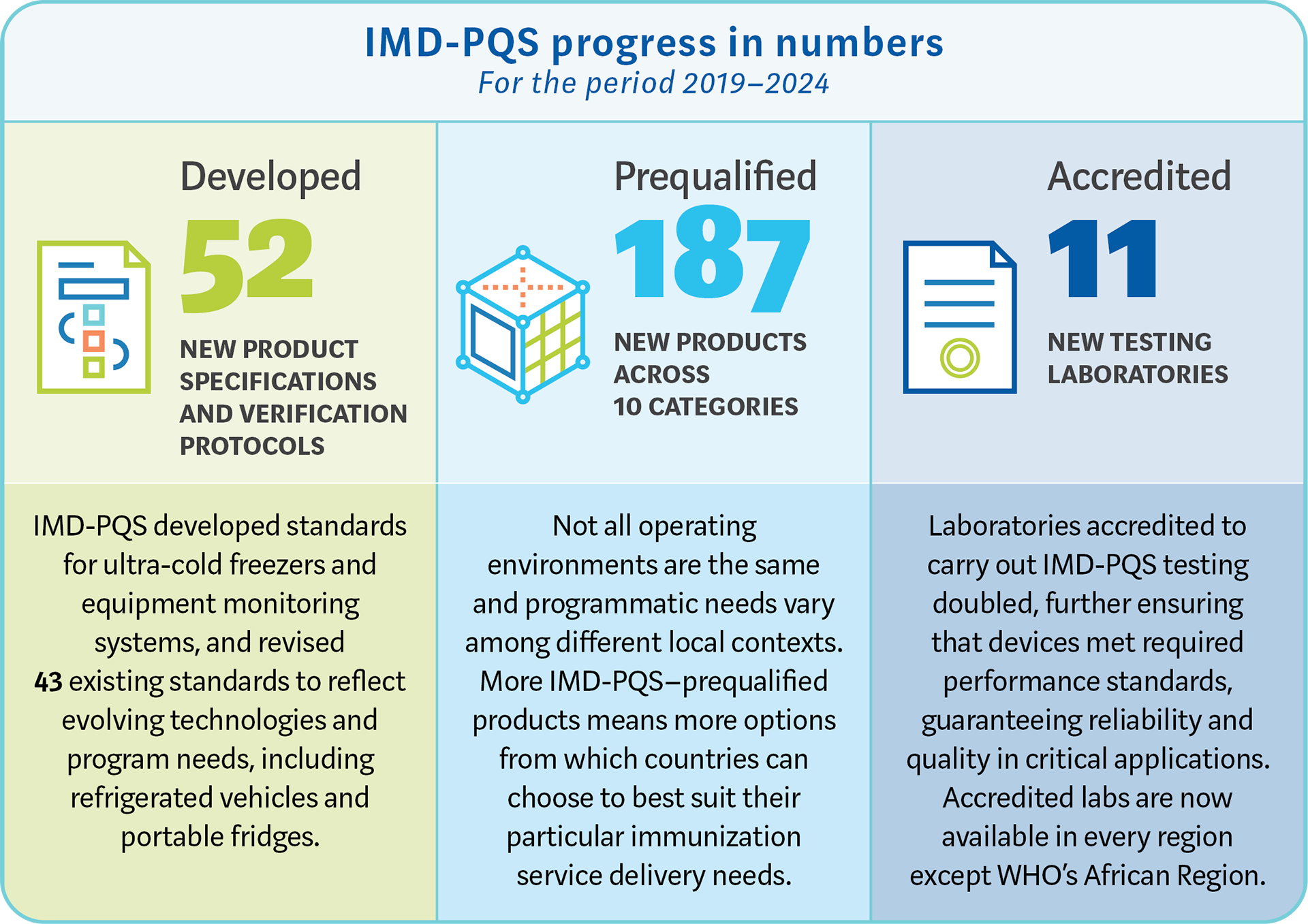
Note: the data and statistics correspond to 2023. Please see the PowerPoint and Strategy above for 2024/2025 statistics.
The 2025-2030 Strategy details WHO Immunization Devices' progress and achievements over the previous five-year period and, crucially, the strategy in place to address the evolving needs of national immunization programmes and new challenges they face. Click the image to download:

2025-2030 Snapshot
IMD-PQS's success & achievements:
Evolving needs of national immunization programmes & new challenges:
Immunization supply chain systems are evolving due to advancements in information and communication technologies, better access to vaccines and immunization data, the growing number of vaccines in immunization schedules, bulkier volumes of new vaccines requiring greater storage capacity, the inclusion of non-vaccine temperature-sensitive health products in the ‘vaccine’ cold chain and higher costs of some newer vaccines.
In addition, IMD-PQS must react to the rapidly changing global landscape in which immunization programmes operate:
Climate change The introduction of vaccines into the cold chain to address expanding disease footprints requires reliable CCE to protect investments and ensure vaccine potency. | |
Pandemic preparedness and response Robust cold chain systems, improved vaccine management processes and the ability to reach broad target populations for vaccine-preventable diseases are crucial. | |
| Global population increase Increases in the global population and the numbers of displaced persons elevates the urgency of immunization and increases the challenges of delivering vaccines. |
To continue to be successful in the future, WHO Immunization Devices will need to prequalify products and devices that keep pace with these changes and ensure the availability of devices that fulfill their evolving vaccine transport and storage needs.