Safety of COVID-19 Vaccines
April 13, 2021
Countries around the world are rolling out COVID-19 vaccines, and a key topic of interest is their safety. Vaccine safety is one of WHO’s highest priorities, and we’re working closely with national authorities to develop and implement standards to ensure that COVID-19 vaccines are safe and effective.
Ensuring safety

Millions of people have safely received COVID-19 vaccines. All of the approved COVID-19 vaccines have been carefully tested and continue to be monitored.
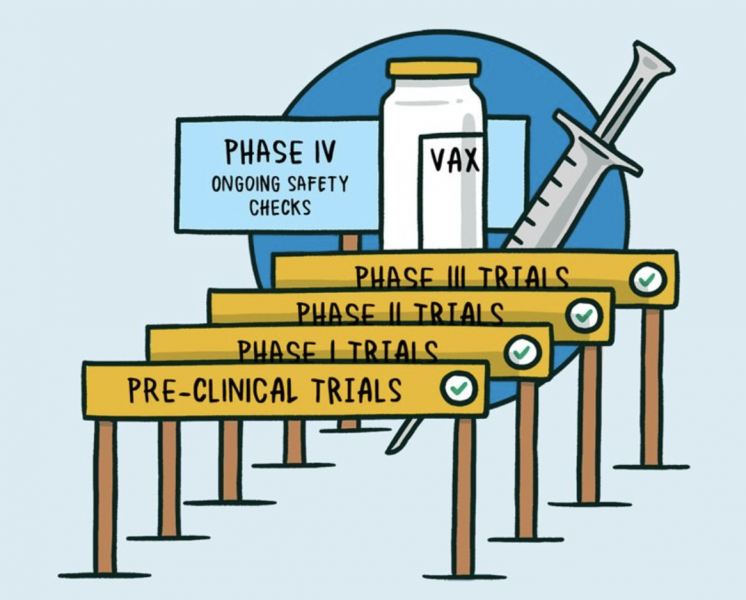
Like all vaccines, COVID-19 vaccines go through a rigorous, multi-stage testing process, including large clinical trials that involve tens of thousands of people. These trials are specifically designed to identify any safety concerns
An external panel of experts convened by WHO analyses the results from clinical trials and recommends whether and how the vaccines should be used. Officials in individual countries decide whether to approve the vaccines for national use and develop policies for how to use the vaccines based on WHO recommendations.
After a COVID-19 vaccine is introduced, WHO supports work with vaccine manufacturers, health officials in each country and other partners to monitor for any safety concerns on an ongoing basis.
New vaccine technology
Some COVID-19 vaccines have been developed with an approach that uses messenger RNA (mRNA). The mRNA vaccine technology has been studied for over a decade, including in the development of vaccines for Zika, rabies and influenza.
These mRNA vaccines have been rigorously assessed for safety, and clinical trials have shown that they provide a long-lasting immune response. mRNA vaccines are not live virus vaccines and do not interfere with human DNA. For more information on mRNA vaccines, see WHO’s explainer on the different types of COVID-19 vaccines.