Nigerian health workers take country’s first COVID-19 vaccine
April 6, 2021
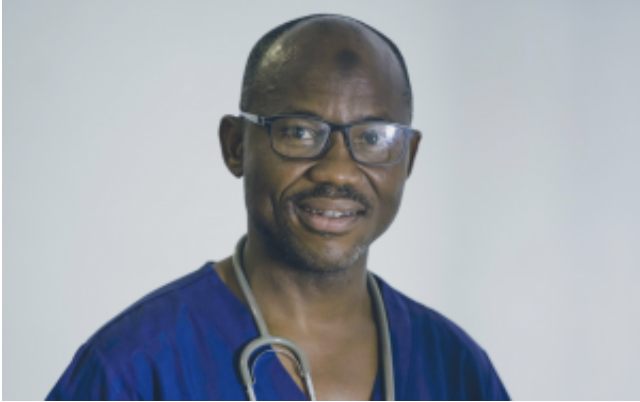
After Yunusa Thairu became one of Nigeria’s earliest COVID-19 vaccine recipients, his mother received a text message that her son had taken the vaccine and something bad might happen to him.
“She got super worried and gave me a call,” the doctor recalls. “I had to drive down to go see and assure her that I am fine, [that] there is nothing wrong with taking the vaccine. Upon seeing how healthy I was, she agreed to take the vaccine when it is available.”
Her son, a medical consultant at the United Nations Nigeria Isolation Center at Durumi in Abuja and with the University of Abuja Teaching Hospital Gwagwalada, is among the 1 million health workers the Government of Nigeria is targeting in the first phase of its nationwide rollout of COVID-19 vaccinations that begin this month.
“Things like this give me joy, taking the lead and others following,” says Dr Thairu. “This pandemic has impacted my work life in so many ways—I see it as a calling on the brave ones to come out and get counted.”
Like Dr Thairu, many Nigerians are eager for the vaccine.
“I am excited about this vaccine,” says Patience Peter. “Finally, we can be at ease. When everyone takes it, we will not have worries of getting infected or losing any loved one. My friends lost their parents and loved ones but it has not affected me directly, it is still sad to see people losing their lives to something that can be prevented. I cannot wait to get vaccinated.”
Included along with the front-line health workers in the first phase of the national vaccination campaign are support staff and other health workers as well as first responders in the military, paramilitary and other security agencies, including immigration services.
Once the health workers are vaccinated, the second phase of the roll out will target adults aged 50 and older (with or without an underlying disease) but starting with those aged 60 and older. Phase 3 will accept anyone aged 18–49 with underlying disease (such as hypertension, diabetes, lung disease, cancers and heart conditions). And the fourth phase will expand to anyone in that age group without any underlying disease condition.
“A lot of demand generation and sensitization of critical stakeholders have gone into the preparedness towards the vaccine rollout,” stresses Dr Faisal Shuaib, Executive Director of the National Primary Health Care Department Agency. His agency and partner agencies, including the World Health Organization (WHO), have deployed electronic self-registration and house-to-house registration for the vaccination campaign across the 36 states and Federal Capital Territory.
Dr Shuaib also emphasizes the “assurances and subsequent certification from the National Agency for Food and Drug Administration and Control” that the AstraZeneca COVID-19 vaccine that the nationwide campaign is beginning with “is safe for use”.