Title
Why
The laboratory must learn from every nonconformity. Acting upon detection of a nonconformity with a procedure following the plan-do-check-act cycle leads to automatic continual improvement following every nonconformity.
What
A nonconformity is any occurrence that is not according to rules and/or expectations. Examples are deviation of critical environmental or equipment parameters, accidents, wrongly packed samples, stock outs, etc. The WHO Laboratory Quality Management System (LQMS) handbook contains information on nonconformity management (called “Occurrence Management” in the LQMS handbook); this is provided in the right-hand column.
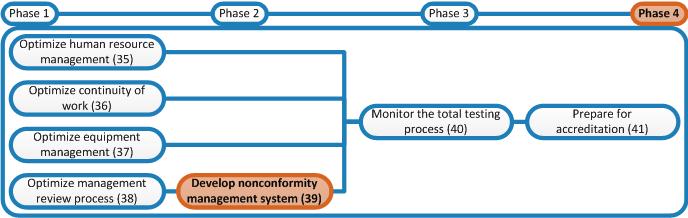
Write an SOP for Nonconformity Management. Appendixes of this SOP are the nonconformity form and the tally list.
For solving nonconformities in a way that leads to continuous improvement the following procedure must be carried out:
- Record every nonconformity on a standardized nonconformity form. A template for such a form is provided in the right-hand column. This form must provide good guidance because the nonconformity can be reported by any staff member; first the nonconformity needs to be described including potential causes. The person reporting the nonconformity must also add his/her name on the form. The form should then show the command "Give this form to the laboratory manager".
- The laboratory manager must subsequently:
- Estimate the severity of the consequences of the nonconformity and indicate this on the form.
- Perform an analysis to investigate what caused the nonconformity. For this the laboratory manager should ask assistance of the person responsible for the laboratory unit in which the nonconformity occurred, and the laboratory manager can ask the reporter of the nonconformity to think along.
- Formulate corrective actions and preventive actions based on the findings from the cause analysis to solve the nonconformity and prevent the same nonconformity from occurring again in the future. If necessary and possible also control steps must be formulated that can detect the nonconformity if it occurs again.
- Timely implementation of the corrective and preventive actions and control steps must be ensured by formulating SMART action points on the nonconformity form, indicating the time-interval (including deadline) in which the nonconformity must be solved/corrected and the name(s) of the person(s) that has/have to implement the action points.
- Below the section where the action points are written the laboratory manager has to indicate if effectiveness of the corrective and preventive actions has to be evaluated. The form must then show the command "Give this form to the Quality Officer".
- The laboratory manager has to discuss the nonconformity including the ways of solving/correcting it in a weekly staff meeting. The persons assigned with the tasks to implement action points must be notified.
- Once the form is given to the Quality Officer he/she has to include the action points in the minutes of the weekly staff meeting to monitor and ensure their implementation. If the laboratory manager has indicated that effectiveness of the corrective and preventive actions must be evaluated, the Quality Officer has to make a tally list for the nonconformity, see below.
- Until the actions points are completed the Quality Officer must not archive the form. Once all action points have been implemented the nonconformity form must be signed and dated by the laboratory manager and subsequently archived in a new folder entitled "Nonconformities".
It is important that a nonconformity form is filled-out for every nonconformity identified. Every staff member should be aware of this as every staff member can help to improve the laboratory services. To increase chances that nonconformities are always recorded on the forms it is crucial that the form is kept simple and that the form can be filled-out in a short time by the person notifying the nonconformity.
A tally list can be used to see if corrective and preventive actions of previous nonconformities have worked. In this case a list is made with a description of the nonconformity that is supposed to be solved/corrected. A template for such a list is provided in the right-hand column. These lists can be placed on a location that is accessible to all staff members, such as the notice board. In a weekly staff meeting staff members are informed about the new lists and are requested to tally the nonconformity when they detect it. If, after implementation of the corrective and preventive actions, the occurrence of the nonconformity is still tallied by staff members on this list, the laboratory manager knows that the corrective and preventive actions were not completely effective.
Tally lists are also very useful for nonconformities that happen more often. For example: Request Forms may not always be filled-out completely. It is too laborious to complete a nonconformity form for every single time this happens. In this case the laboratory can use a tally list on which the occurrence of the nonconformity can be tallied.
How & who
Quality Officer in collaboration with the Laboratory Manager:
- Translate the above description into an SOP for Nonconformity Management together. Follow the protocol for writing a Procedure SOP of the Master SOP and use the template for a Procedure SOP attached to the Master SOP.
- Review and authorize the SOP in accordance with the SOP for Document Control.
- Once finalized and effective, add the SOP to the Read and Understand List and indicate that all staff members have to read the SOP.
- Present the SOP in a weekly staff meeting. Educate staff members about the importance of recording every nonconformity, how the procedure of solving/correcting nonconformities goes, and instruct them on the use of nonconformity forms and tally forms. Explain where staff members can find empty nonconformity forms.
- Monitor if staff members start to report and record nonconformities. If you receive no filled-out nonconformity forms, stress the importance of recording nonconformities again in a weekly staff meeting, until you start receiving filled-out forms.
Laboratory Manager:
- Adapt the Authorization Matrix to include the tasks and responsibilities described in the SOP for Nonconformity Management for the different positions in the laboratory.