BARYCELA inj.
Varicella
Product Details
WHO Product ID
FVP-P-406
Status
Prequalified
Date of prequalification
Vaccine type
Varicella
Abbreviated Name
VAR
Commercial Name
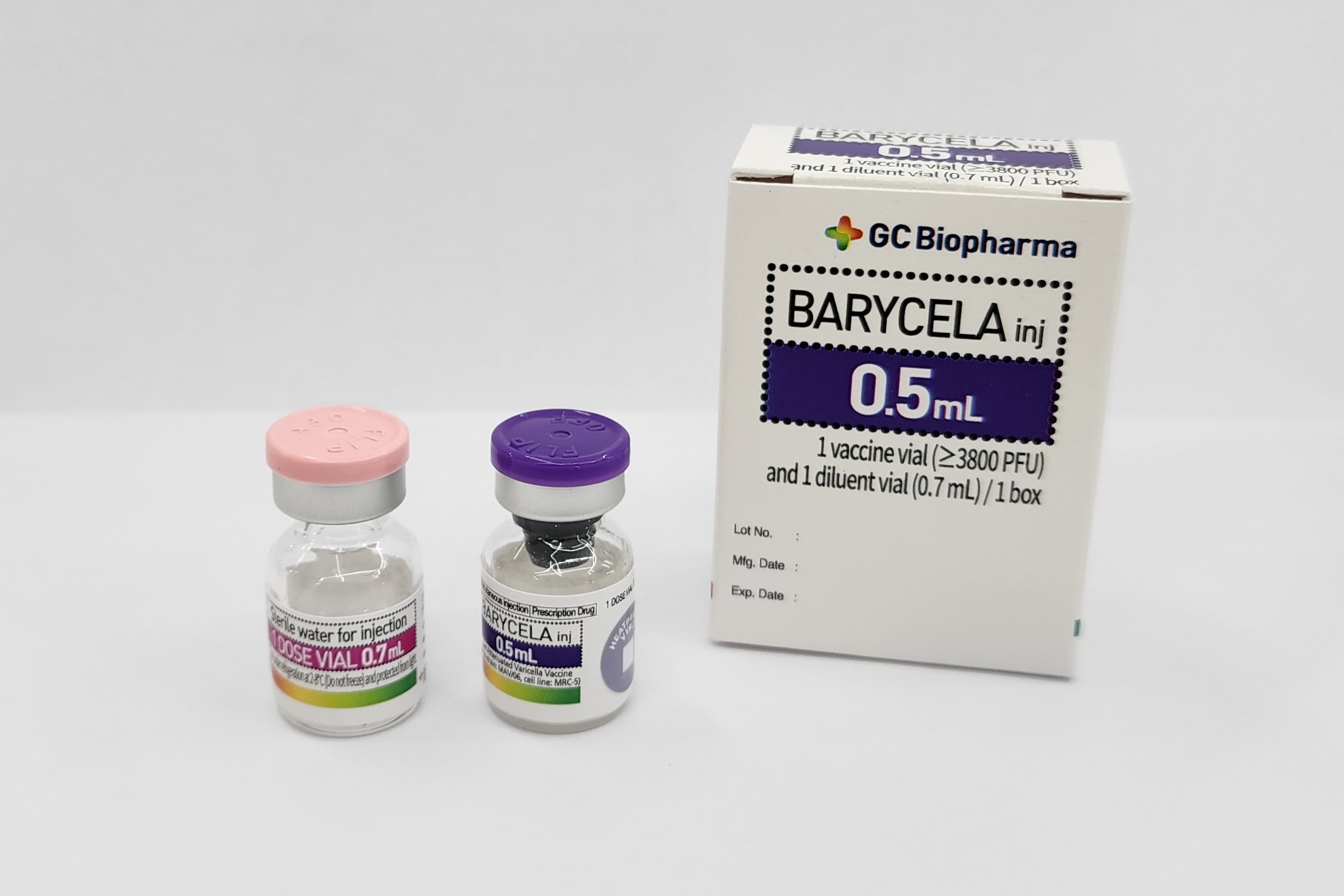
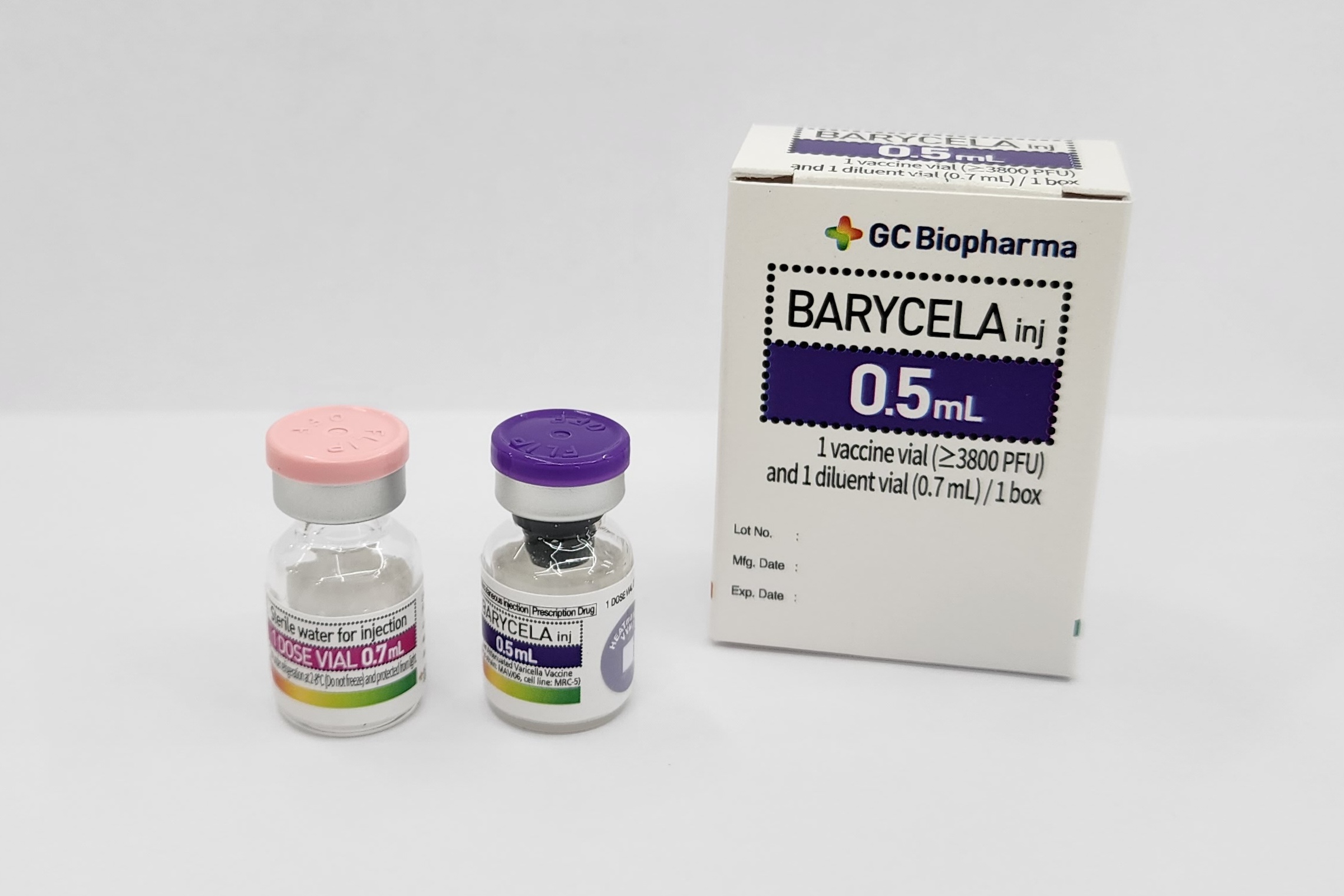
BARYCELA inj.
PQ Holder
GC Biopharma Corporation
303 Bojeong-dong, Yongin
Giheung-gu,
446-770
Republic of Korea
Responsible NRA
Ministry of Food and Drug Safety (MFDS)
Product description
Pharmaceutical Form
Lyophilised active component to be reconstituted with excipient diluent before use
Presentation
Vial
Number of doses
1
Route of administration
Subcutaneous
Vaccine Vial Monitor
Type 7
Multidose Vial Policy
Not applicable
Shelf life (months)
24 months
Storage temperature
2 - 8°C
Diluent
Water for injection
Preservative
None
Remarks
Diluent shelf-life is 36 months at 2 to 8°C
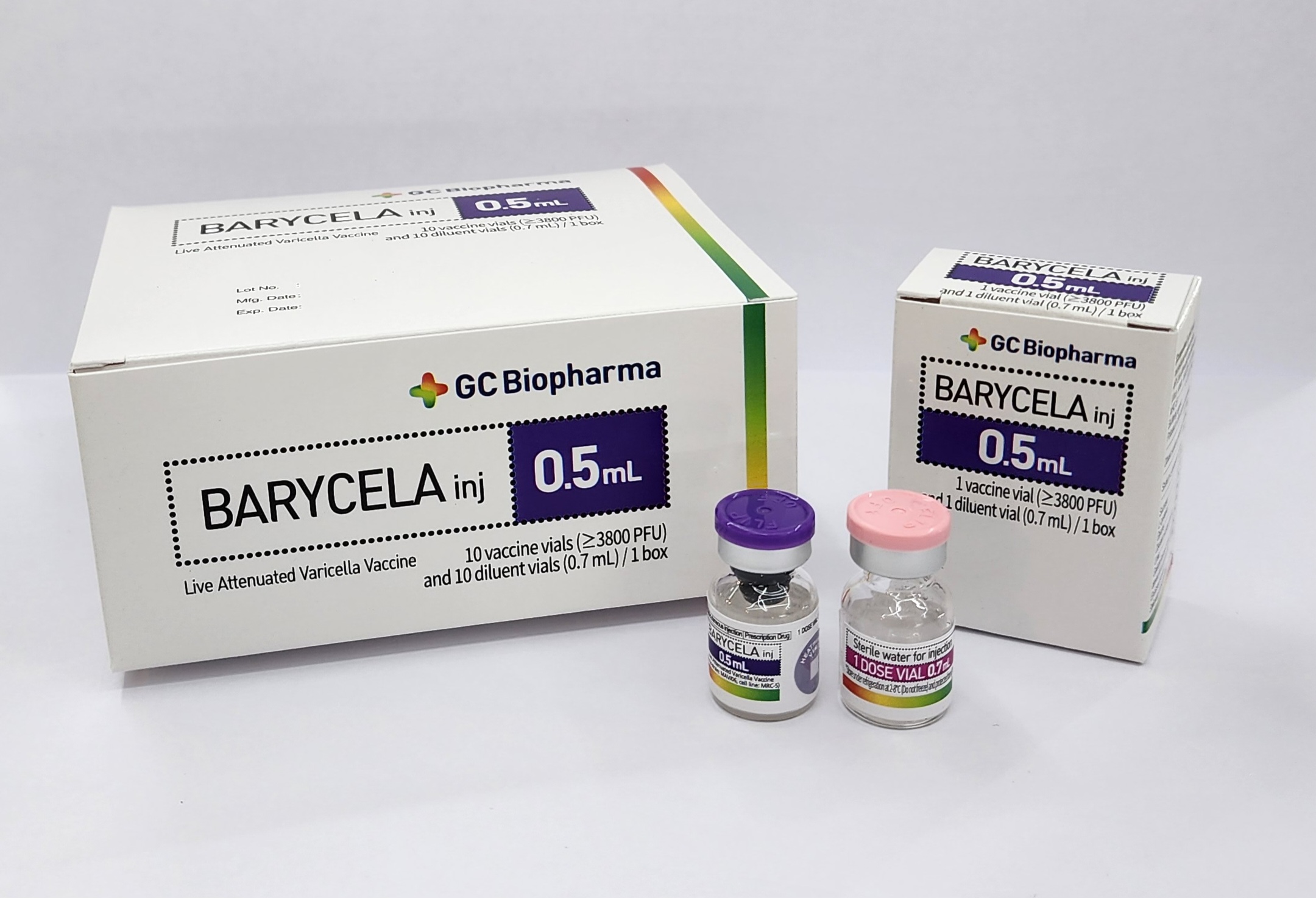
Product Packaging
Secondary Container
Shipping Container
Component Packed
Active (Vaccine) and Diluent
Total doses
10
Description
Carton box containing 5 lyophilized vaccine vials and 5 diluent vials.
Dimensions
8.85 x 4.55 x 3.65 cm
Cold Chain Volume (cm³/dose)
29.4
Component Packed
Active (Vaccine)
Total doses
10
Description
Carton box containing 10 lyophilized vaccine vials.
Dimensions
8.85 x 4.55 x 3.65 cm
Cold Chain Volume (cm³/dose)
14.7
Component Packed
Diluent
Total doses
10
Description
Carton box containing 10 diluent vials.
Dimensions
8.85 x 4.55 x 3.65 cm
Cold Chain Volume (cm³/dose)
14.7