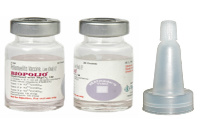
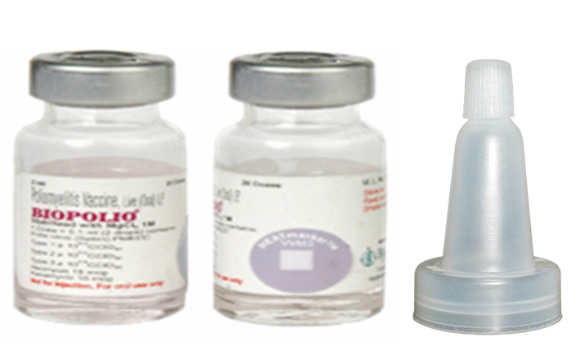
BIOPOLIO
Poliomyelitis vaccines (trivalent live, oral, innactivated, type 1,2, 3)
Product Details
WHO Product ID
FVP-P-291
Status
Prequalified
Date of prequalification
Vaccine type
Poliomyelitis vaccines (trivalent live, oral, innactivated, type 1,2, 3)
Abbreviated Name
tOPV
Commercial Name
BIOPOLIO
PQ Holder
Bharat Biotech International Limited
Sy. No. 230 231 & 235 Genome Valley Turkapally Shamee
rpet Mandal Medchal-Malkajgiri District
rpet Mandal Medchal-Malkajgiri District
Hyderabad,
Telangana
500 078
India
Responsible NRA
Central Drugs Standard Control Organization (CDSCO)
Product description
Bulk supplier
PT Bio Farma (Persero)
Pharmaceutical Form
Liquid: Ready to use
Presentation
Vial
Number of doses
10
Route of administration
Oral
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be used in subsequent immunization sessions (up to 28 days) if the conditions outlined in the WHO Policy Statement are met.
Shelf life (months)
24 months
Storage temperature
-20°C
Diluent
N/A
Preservative
None
Remarks
Ten dose container image not yet provided. Container image shows the twenty dose presentation (same vial dimensions as the 10 dose presentation). There is no routine production for this vaccine.
Product Packaging
Secondary Container
Tertiary Container
Component Packed
Active (Vaccine)
Total doses
25
Description
Carton of 25 vials (250 doses) [Dimensions 11.0 x 11.0 x 3.5 cm]
Dimensions
11.0 x 11.0 x 3.5 cm
Cold Chain Volume (cm³/dose)
1.69