Title
Why
In phase 1 SOPs already needed to be developed for all examinations performed in the laboratory and for operation and maintenance of all equipment present in the laboratory. However, examinations are preceded and followed by many other procedures to enable the primary process of the laboratory to function properly. To be able to assure quality performance of the laboratory these additional routinely performed procedures also need to be standardized. This ensures that every procedure is always performed in the same, correct way, which in turn helps to ensure that the core process of the laboratory can perform properly and is quality assured.
What
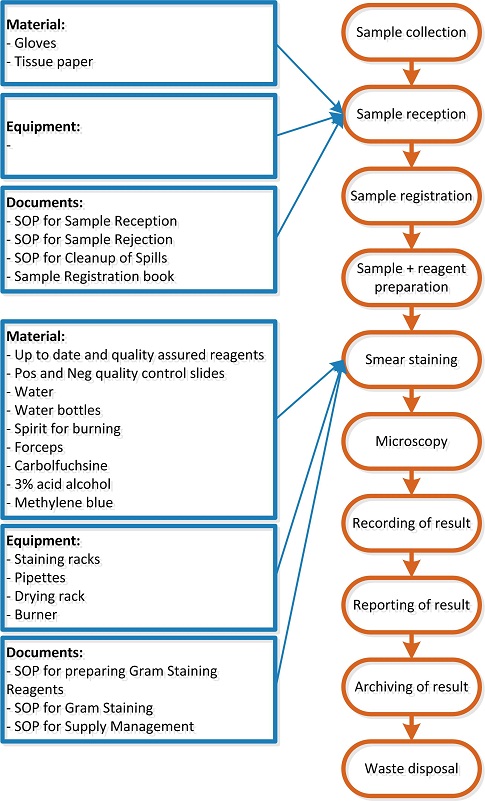
Earlier in phase 2 flowcharts needed to be made of all the routinely performed procedures in each unit/section of the laboratory. These flowcharts are used to make an inventory of all the SOPs needed. For each process step in a flowchart the input requirements (materials and equipment) need to be determined and subsequently the SOPs are listed that are needed to ensure correct and standardized execution of the process step and good quality input requirements.
How & who
Quality Officer in cooperation with unit/section heads and the Laboratory Manager:
- Collect all the flowcharts developed earlier in phase 2.
- Determine in each flowchart for each step which SOPs are needed. Do this according to the following procedure:
- Determine which equipment is needed in each step
- Determine which materials besides equipment are needed in each step
- Determine which SOP(s) is/are needed to perform the step and determine which SOPs are needed for preparation and use of specific materials and equipment in each step. Also think of safety SOPs.
See the image below in which the above steps are performed for two process steps of the example Gram Stain procedure (please note that the input requirements shown in this figure may not be complete as they serve merely as example).
- Quality Officer: make a complete overview of all the SOPs that need to be written. Then continue with the next activity.