Product overview
Vaccine type
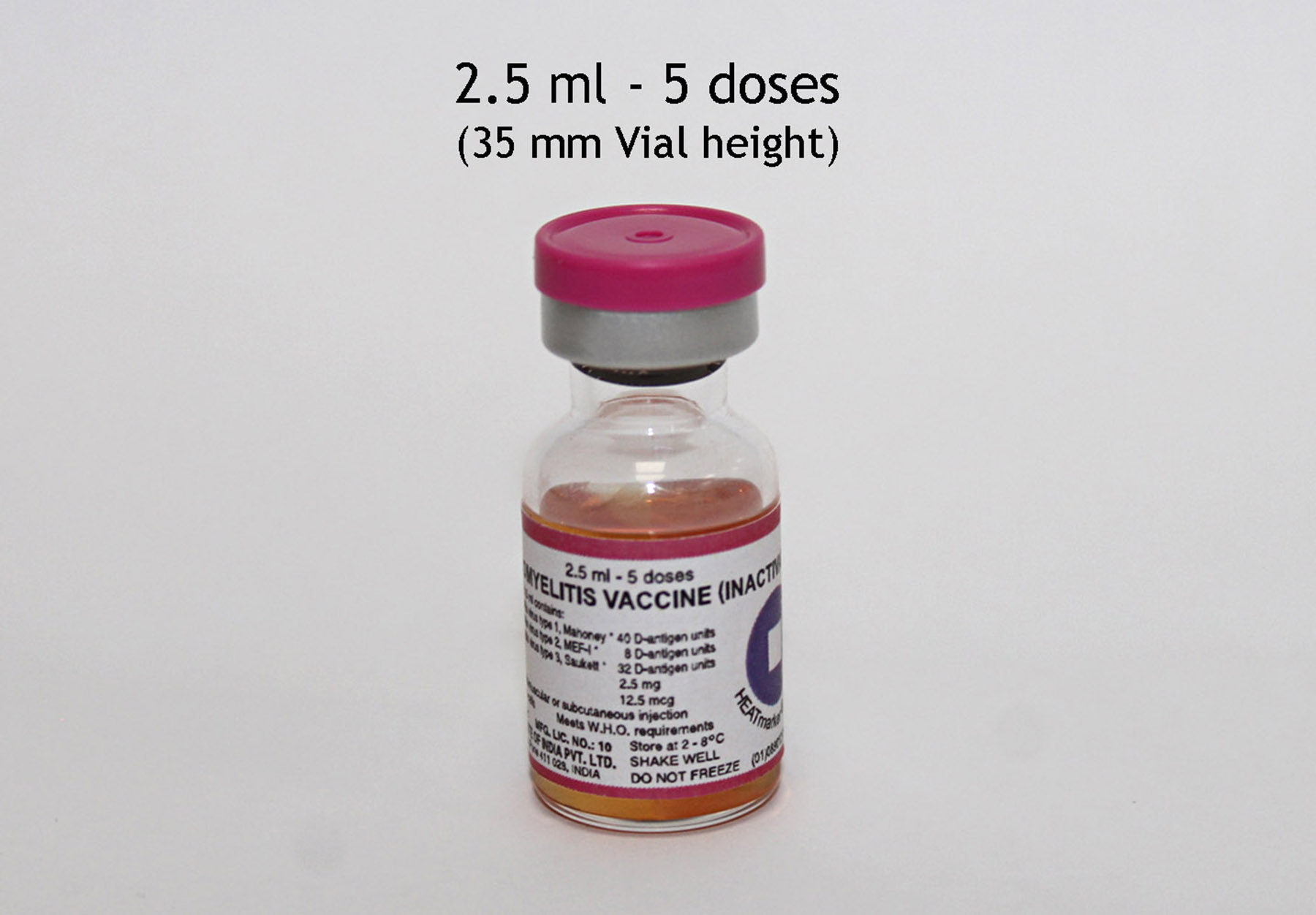
Polio Vaccine - Inactivated (IPV)
Commercial Name
Poliomyelitis Vaccine (Inactivated)
Manufacturer
Serum Institute of India Pvt. Ltd.
Responsible NRA
Central Drugs Standard Control Organization
Country
India
URL
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
5
Diluent
Not Applicable
Route of Administration
Intramuscular or Subcutaneous
Shelf Life
36 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 11
Secondary Packaging
Carton of 50 vials/ 250 doses [Dimensions: 18.5 x 9.5 x 5.0 cm]
Tertiary Packaging
Box containing 24 cartons of 50 vials (1200 vials/ 6000 doses) [Dimensions: 60 x 48 x 36 cm]
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be kept for use in subsequent immunization sessions (up to a maximum of 28 days) provided the conditions outlined in the WHO Policy Statement: The use of opened multi-dose vials of vaccine in subsequent immunization sessions are met.
Vaccine Preservative
2-phenoxyethanol
Vaccine Preservative Concentration
2.5 mg/dose
Cold Chain Volume
3.515 cm3/dose (in secondary packaging)