Product overview
Vaccine type
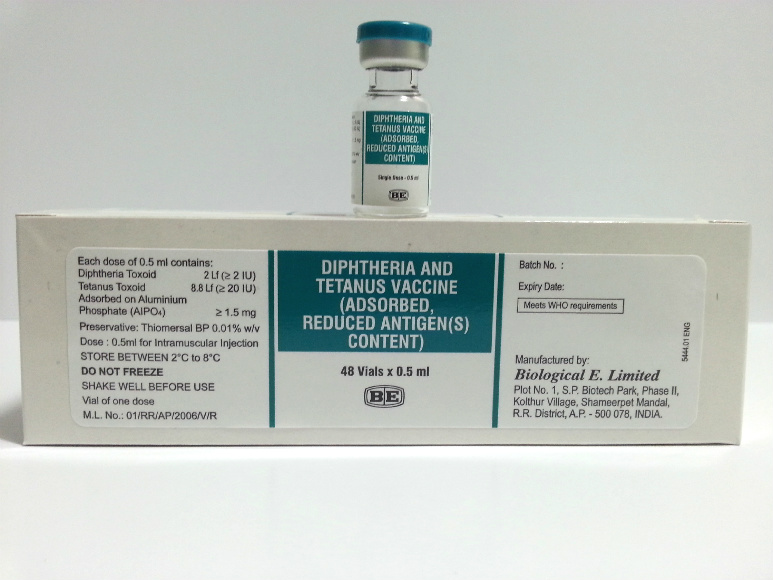
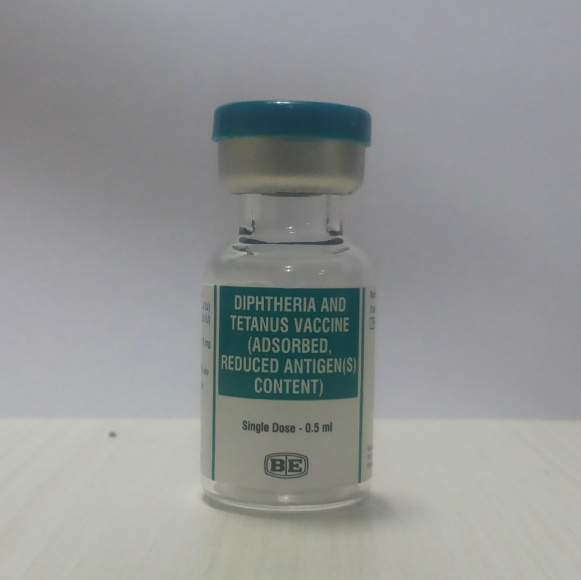
Diphtheria-Tetanus (reduced antigen content)
Commercial Name
None used on labelling for supply through UN agencies. Also marketed with labelled commercial name BE Td
Manufacturer
Biological E. Limited
Responsible NRA
Central Drugs Standard Control Organization
Country
India
URL
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
1
Diluent
Not Applicable
Route of Administration
Intramuscular
Shelf Life
36 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 30
Secondary Packaging
Carton of 48 vials (48 doses) [Dimensions 14.5x10.8x4.5 cm]
Tertiary Packaging
Box of 24 cartons of 48 vials (1152 vials) [Dimensions 58.5x47.0x39.0 cm]
Multidose Vial Policy
Not Applicable.
Vaccine Preservative
thiomersal
Vaccine Preservative Concentration
0.005 % w/v
Cold Chain Volume
14.68 cm3/dose (in secondary packaging)