Product overview
Vaccine type
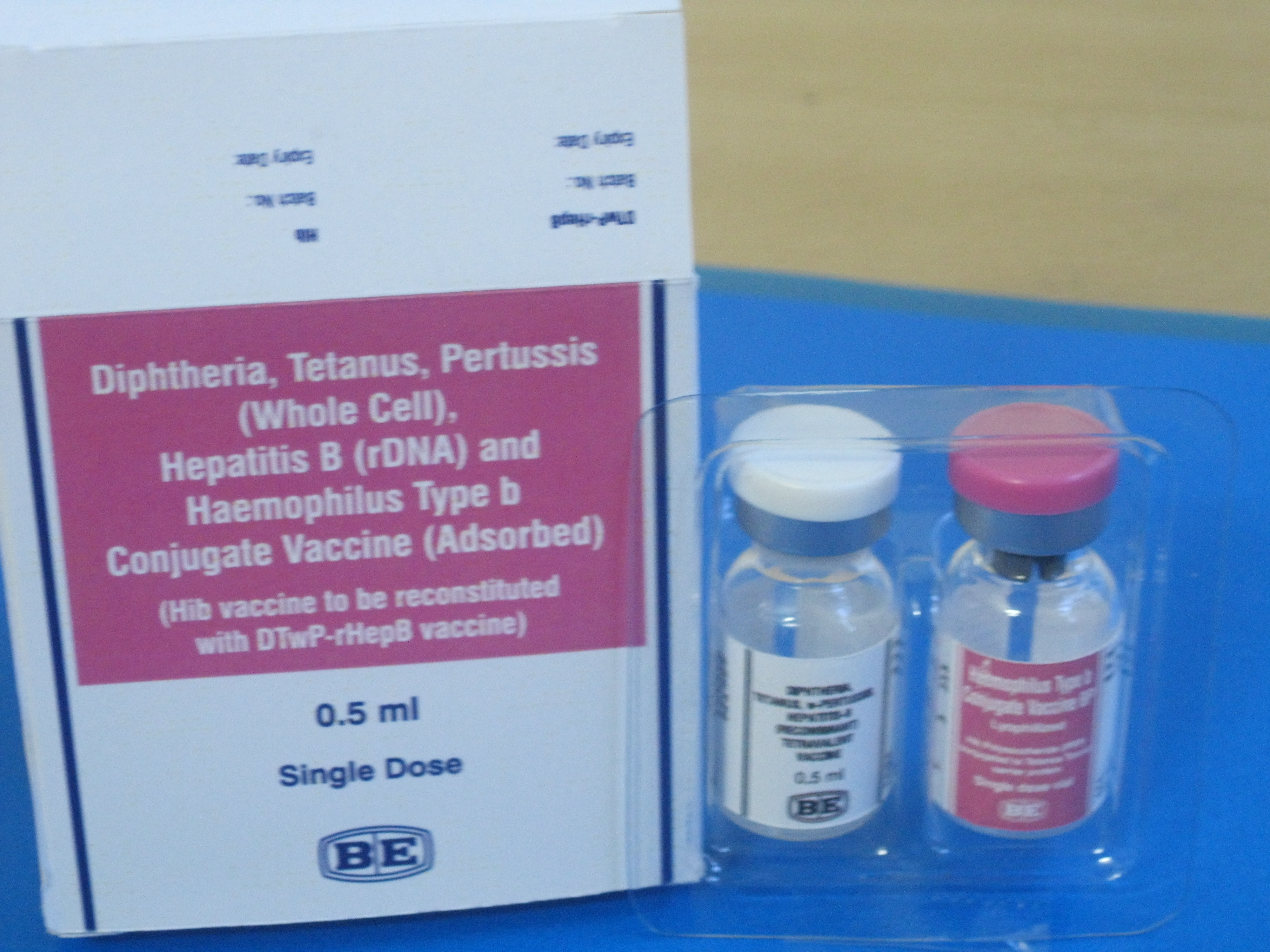
Diphtheria-Tetanus-Pertussis (whole cell)-Hepatitis B-Haemophilus influenzae type b
Commercial Name
None used on labelling for supply through UN agencies. Also marketed with labelled commercial name ComBE Five (Reconstituted).
Manufacturer
Biological E. Limited
Responsible NRA
Central Drugs Standard Control Organization
Country
India
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Lyophilised active component to be reconstituted with liquid active component before use
Presentation
Two vial set (active + active)
Number of Doses
1
Diluent
Liquid active component
Route of Administration
Intramuscular
Shelf Life
24 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 14
Secondary Packaging
carton of 216 vials of Hib and 216 vials of DTP-HepB
Multidose Vial Policy
Not Applicable.
Vaccine Preservative
thiomersal
Vaccine Preservative Concentration
0.01% w/v
Cold Chain Volume
58.7 cm3/dose (in secondary packaging)