Product overview
Vaccine type
Influenza, seasonal (Trivalent)
Commercial Name
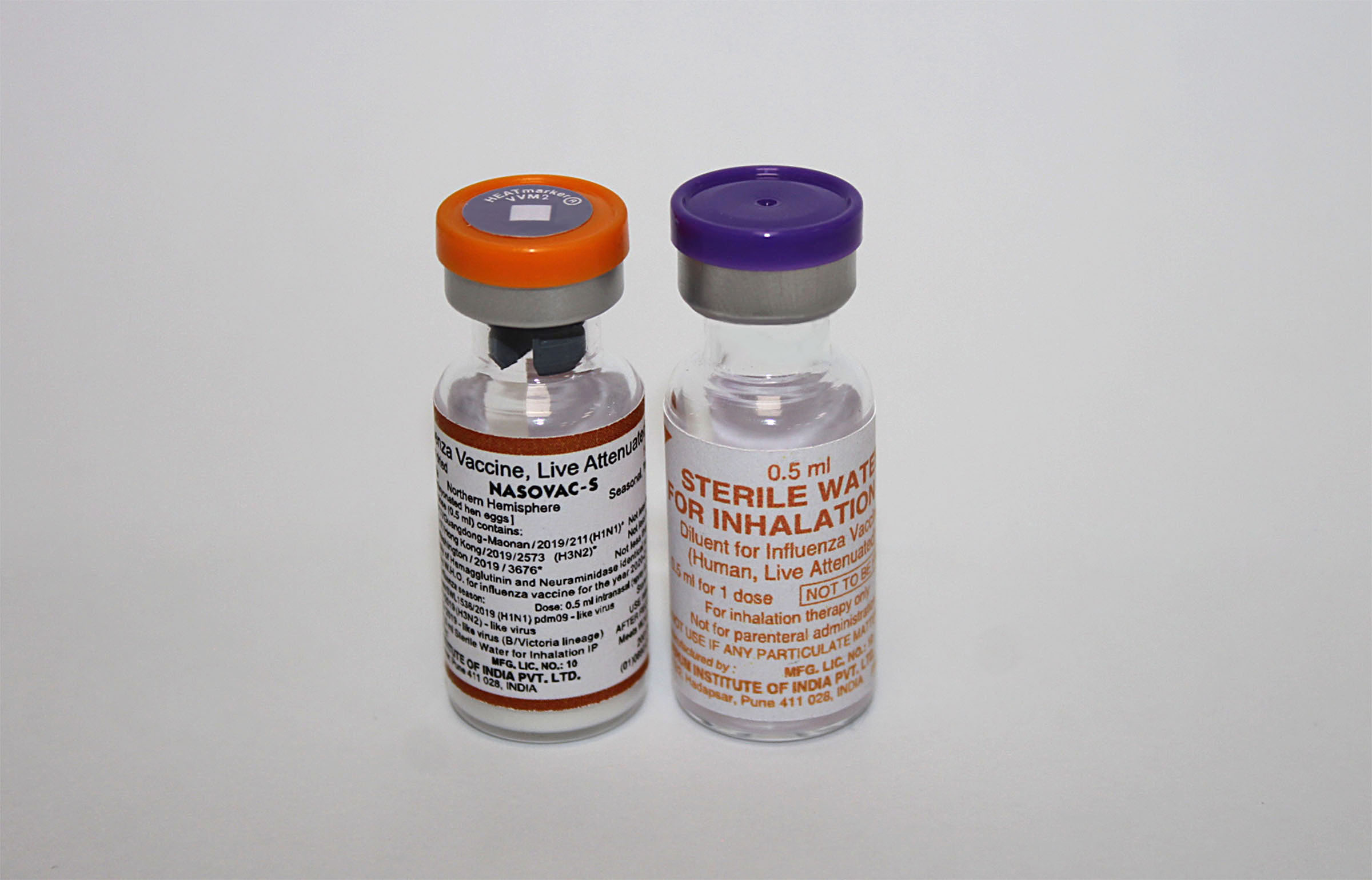
Nasovac-S Influenza Vaccine, Live, Attenuated (Human)
Manufacturer
Serum Institute of India Pvt. Ltd.
Responsible NRA
Central Drugs Standard Control Organization
Country
India
URL
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Lyophilised active component to be reconstituted with excipient diluent before use
Presentation
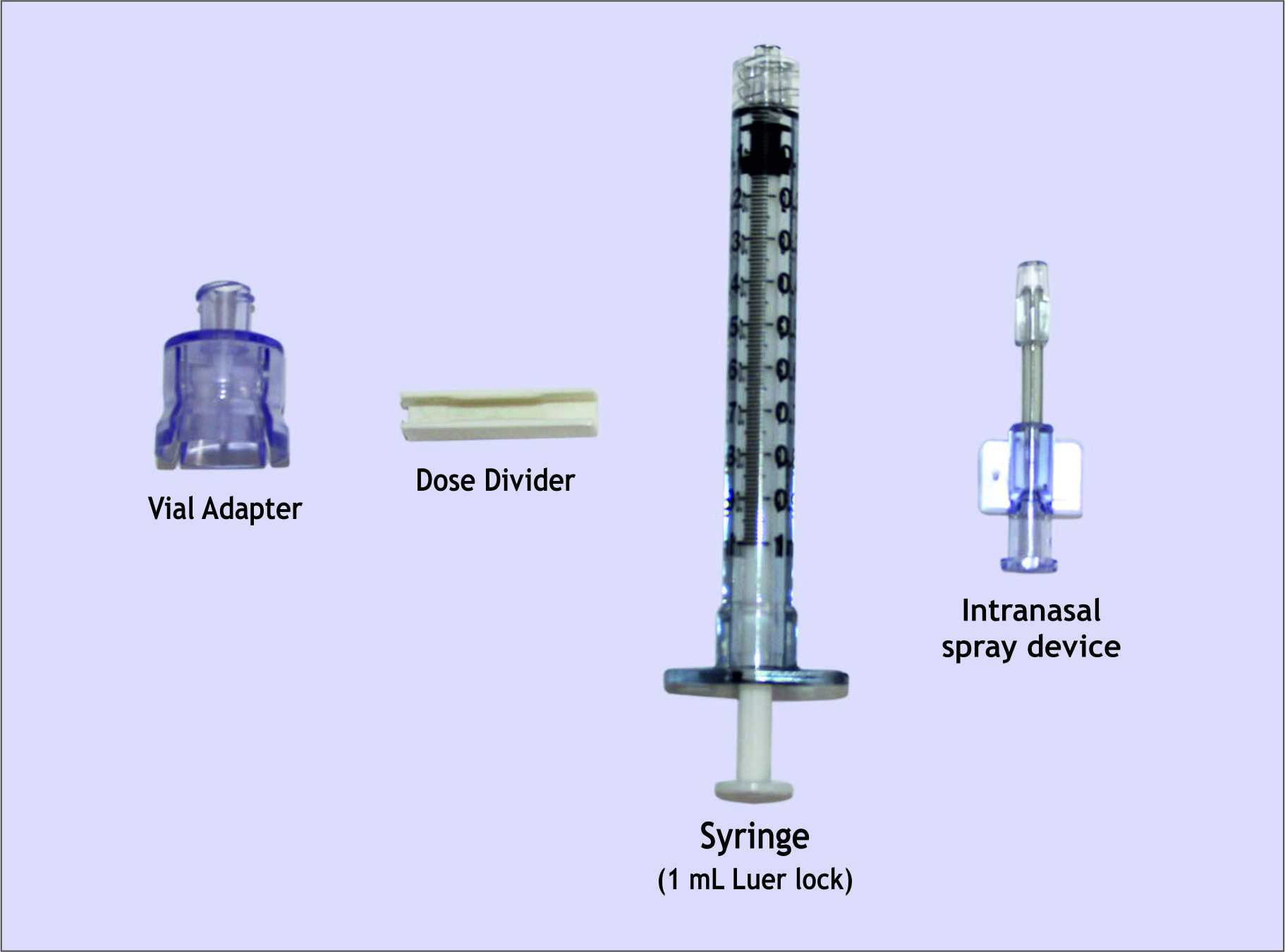
Vial + Ampoule
Number of Doses
1
Route of Administration
Intranasal
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 2
Secondary Packaging
Carton of 50 vials (active)(50 doses) [Dimensions 18.5x9.5x5.0 cm]; See remarks below
Tertiary Packaging
Box of 24 cartons of 50 vials (1200 vials/1200 doses) [Dimensions 60x48x41 cm]
Multidose Vial Policy
Not Applicable.
Vaccine Preservative
none
Cold Chain Volume
17.575 cm3/dose (in secondary packaging)