Product overview
Vaccine type
Influenza, Pandemic (H1N1)
Commercial Name
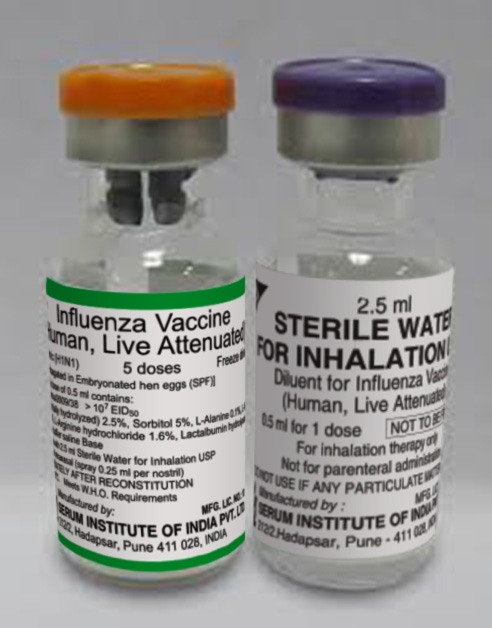
NASOVAC Influenza Vaccine, Live Attenuated (Human) Freeze-Dried
Manufacturer
Serum Institute of India Pvt. Ltd.
Responsible NRA
Central Drugs Standard Control Organization
Country
India
URL
Prequalification
Prequalification Status
Current: No Routine Production
Product description
Pharmaceutical Form
Lyophilised active component to be reconstituted with excipient diluent before use
Presentation
Vial + Ampoule
Number of Doses
5
Route of Administration
Intranasal
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 2
Secondary Packaging
a. Carton of 50 vials (active)(250 doses) [Dimensions 18.5x9.5x5.0 cm]; b. Carton of 50 vials (diluent) (250 doses) [Dimensions 18.5x9.5x5.0 cm] containing 2.5 mL Sterile Water for Inhalation.
Tertiary Packaging
a. Box of 24 cartons of 50 vials (active)(1200 vials/6000 doses) [Dimensions 60x48x41 cm]; b. Box of 24 cartons of 50 vials (diluent)(1200 vials/6000 doses) [dimension 39x32x23 cm]
Multidose Vial Policy
WHO recommends that opened vials of this vaccine should be discarded 6 hours after opening or at the end of the immunization session, whichever comes first.
Vaccine Preservative
none
Cold Chain Volume
3.515 cm3/dose (in secondary packaging)