Product overview
Vaccine type
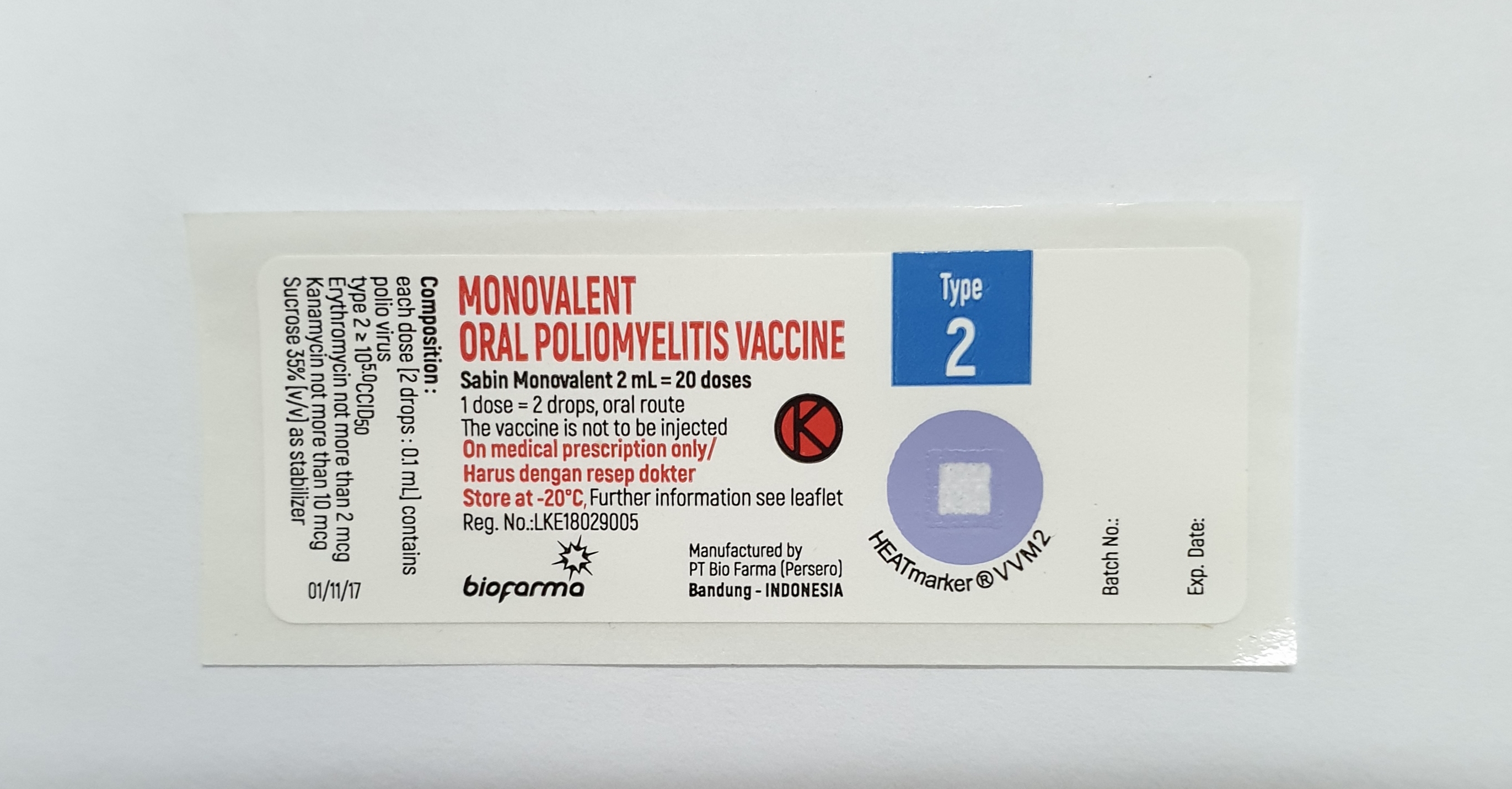
Polio Vaccine - Oral (OPV) Monovalent Type 2
Commercial Name
Monovalent Oral Poliomyelitis Vaccine Type 2
Manufacturer
PT Bio Farma (Persero)
Responsible NRA
National Agency of Drug and Food Control Indonesia
Country
Indonesia
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
20
Diluent
Not Applicable
Route of Administration
Oral
Shelf Life
24 months
Storage Temperature
-20°C
Vaccine Vial Monitor
Type 2
Secondary Packaging
Carton of 50 vials (1000 doses)[Dimensions: 17 x 3.8 x 8.5 cm]
Tertiary Packaging
Type 1. Box containing 2400 vials (48 000 doses) or 2700 vials (54 000 doses)[Dimensions: 65 x 51 x 60 cm] Type 2. Box containing 1500 vials (30 000 doses) or 1400 vials (28 000 doses)[Dimensions: 52 x 52 x 53 cm) Type 3. Box containing 300 vials (36 000 doses)[Dimensions: 52 x 49 x 46 cm]
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be kept for use in subsequent immunization sessions (up to a maximum of 28 days) provided the conditions outlined in the WHO Policy Statement: The use of opened multi-dose vials of vaccine in subsequent immunization sessions are met.
Vaccine Preservative
none
Cold Chain Volume
0.55 cm3/dose (in secondary packaging)