Product overview
Vaccine type
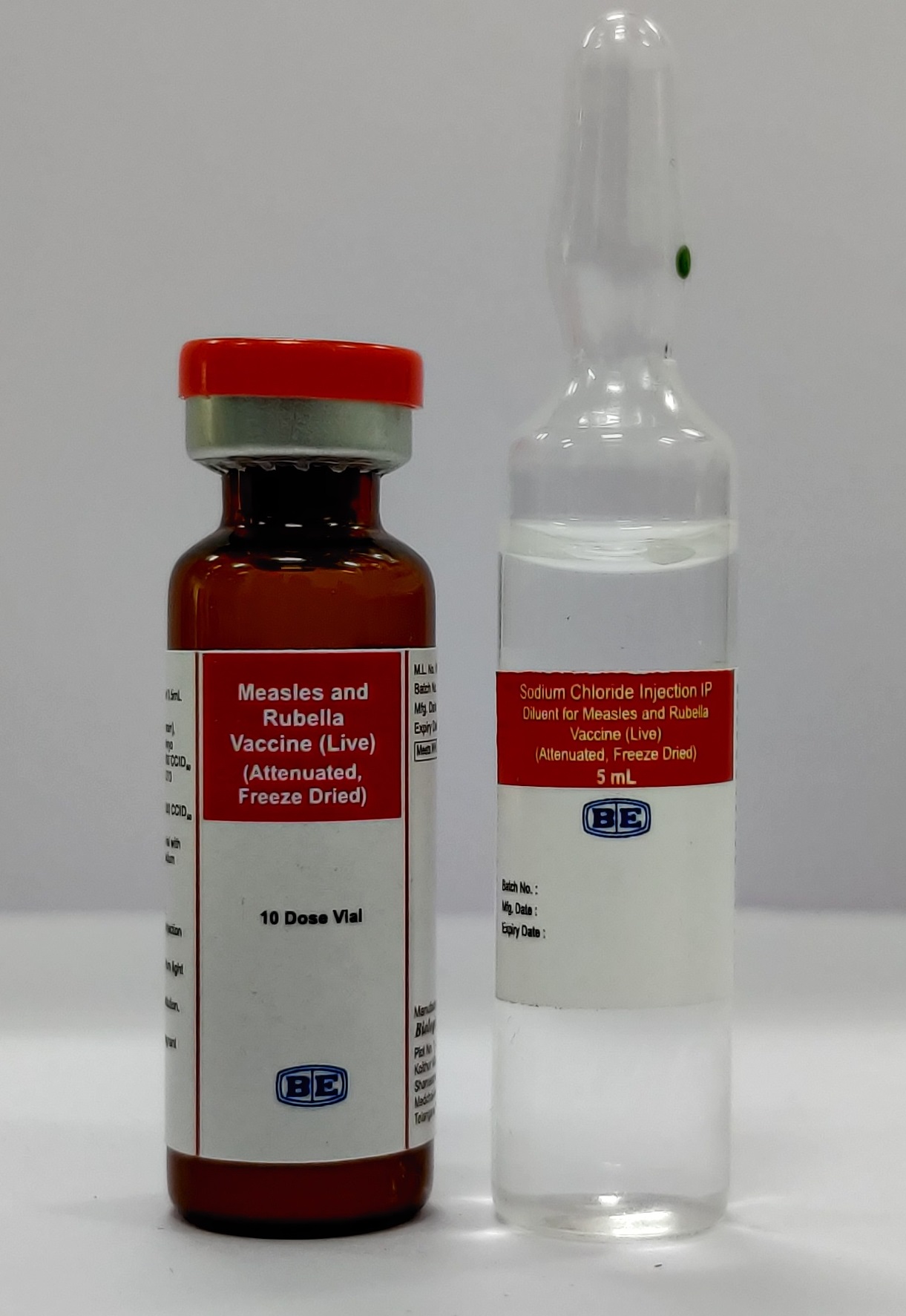
Measles and Rubella
Commercial Name
Measles and Rubella Vaccine (Live) (Attenuated, Freeze Dried)
Manufacturer
Biological E. Limited
Responsible NRA
Central Drugs Standard Control Organization
Country
India
URL
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Lyophilised active component to be reconstituted with excipient diluent before use
Presentation
Vial + Ampoule
Number of Doses
10
Diluent
0.9% Sodium Chloride
Route of Administration
Subcutaneous
Shelf Life
30 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 14
Secondary Packaging
Active: Carton of 50 vials/ 500 doses [Dimensions: 16.5 x 9 x 6 cm]; Diluent: Carton containing 50 ampoules/ 500 doses [Dimensions: 16.2 x 8.4 x 7.8 cm]
Tertiary Packaging
Active: Box containing 24 cartons of 50 vials (1200 vials/12000 doses)[Dimensions: 58.5 x 47 x 39 cm]: Diluent: Box containing 24 cartons of 50 ampoules (1200 amp./ 12000 doses)[Dimensions: 34.3 x 33 x 25 cm]
Multidose Vial Policy
WHO recommends that opened vials of this vaccine should be discarded 6 hours after opening or at the end of the immunization session, whichever comes first.
Vaccine Preservative
none
Cold Chain Volume
Active: 1.78 Diluent: 2.12 cm3/dose (in secondary packaging)