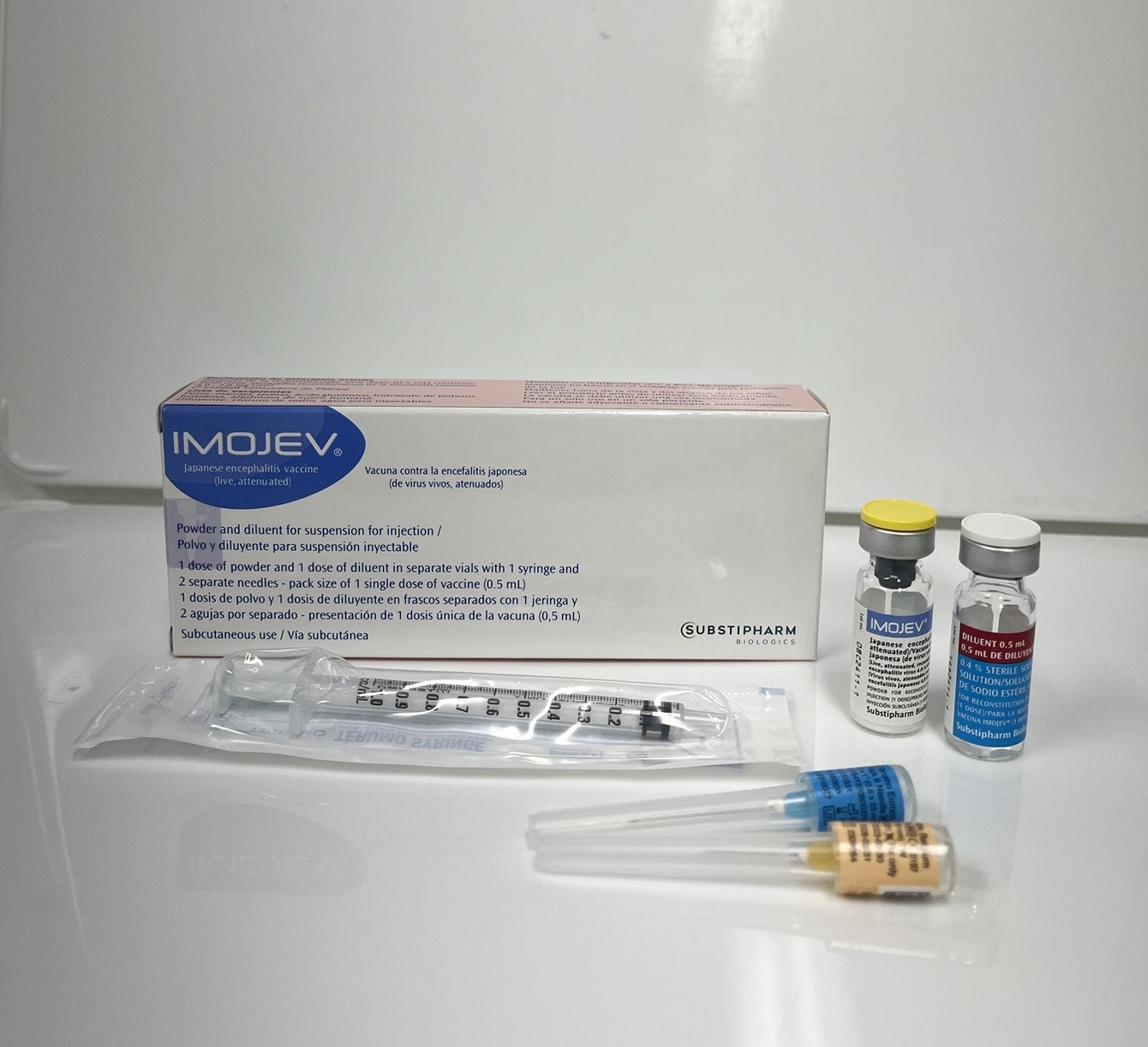
Product overview
Vaccine type
Japanese Encephalitis Vaccine (live, attenuated)
Commercial Name
IMOJEV MD
Manufacturer
Global Biotech Products Co., Ltd
Responsible NRA
Thai Food and Drug Administration
Country
Thailand
URL
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Lyophilised active component to be reconstituted with excipient diluent before use
Presentation
Two vial set (active + excipient)
Number of Doses
4
Diluent
0.9% Sodium Chloride
Route of Administration
Subcutaneous
Shelf Life
36 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 14
Secondary Packaging
Carton of 10 vials (active) (40 doses) [Dimensions 7.4 x 3.1 x 4.3 cm]; Separate carton of 10 vials (diluent) (which can be stored outside cold chain)
Tertiary Packaging
Box of 20 cartons of 10 vials. Insulated shipper box of 18 tertiary cartons (14400 doses)[Dimensions 64 x 69 x 41 cm]
Multidose Vial Policy
WHO recommends that opened vials of this vaccine should be discarded 6 hours after opening or at the end of the immunization session, whichever comes first.
Vaccine Preservative
none
Cold Chain Volume
2.5 cm3/dose (in secondary packaging)