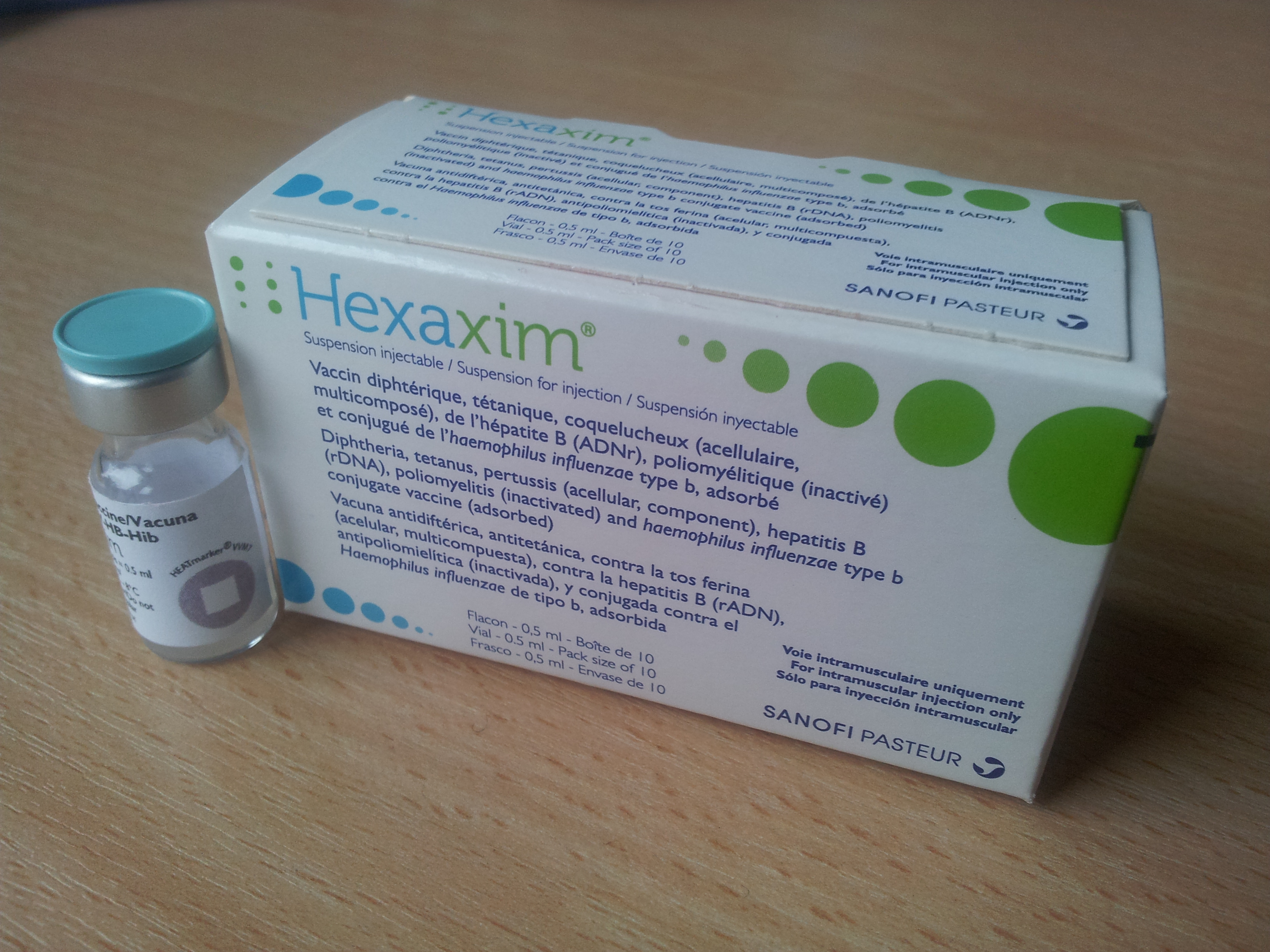
Product overview
Vaccine type
Diphtheria-Tetanus-Pertussis (acellular)-Hepatitis B-Haemophilus influenzae type b-Polio (Inactivated)
Commercial Name
Hexaxim
Manufacturer
Sanofi Winthrop Industrie
Responsible NRA
European Medicines Agency
Country
Netherlands
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
1
Diluent
Not Applicable
Route of Administration
Intramuscular
Shelf Life
36 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 7
Secondary Packaging
Carton of 10 vials [Dimensions 7.7x3.5x4.2 cm]
Tertiary Packaging
Expanded polystyrene box containing 384 cartons (of 10 vials) [50 X 40 X 38 cm] followed by Shipper [70.6 X 61.2 X 60 cm]
Multidose Vial Policy
Not Applicable.
Vaccine Preservative
none
Cold Chain Volume
11.32 cm3/dose (in secondary packaging)