Product overview
Vaccine type
Hepatitis B
Commercial Name
Hepatitis B Vaccine (rDNA) (Adult)
Manufacturer
Serum Institute of India Pvt. Ltd.
Responsible NRA
Central Drugs Standard Control Organization
Country
India
URL
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
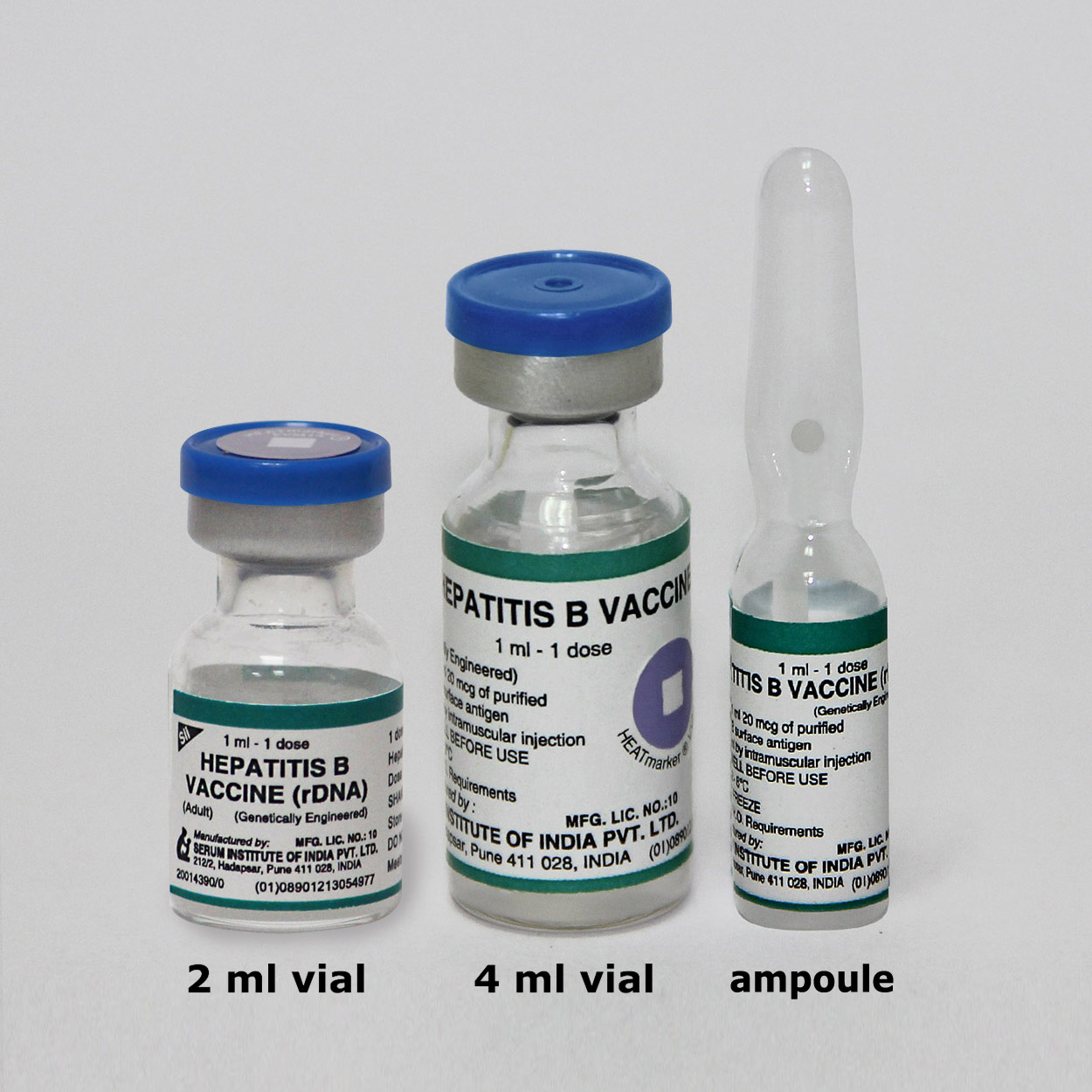
Presentation
Ampoule or Vial
Number of Doses
1
Diluent
Not Applicable
Route of Administration
Intramuscular
Shelf Life
36 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 30
Secondary Packaging
a. Carton of 50 vials (50 doses) [Dimensions 18.5 x 9.5 x 5.0 cm (4 ml)]; b. Carton of 50 vials (50 doses)[Dimensions: 18.5 x 9.5 x 4.0 cm (2 ml)]; c. Carton of 50 ampoules (50 doses) [Dimensions: 14.5 x 6.0 x 7.0 cm]
Tertiary Packaging
a. Box of 24 cartons of 50 vials (1200 vials/1200 doses) [Dimensions: 60 x 48 x 36 cm]; b. Box of 36 cartons of 50 vials (1800 vials/ 1800 doses) [Dimensions: 60 x 48 x 48.5 cm); c. Box of 36 cartons of 50 ampoules (1800 ampoules/1800 doses) [Dimensions: 60 x 48 x 41 cm]
Multidose Vial Policy
Not Applicable.
Vaccine Preservative
thiomersal
Vaccine Preservative Concentration
0.005%
Cold Chain Volume
a. 17.575 (40 mm vial); b.14.06 (30 mm vial) c.12.18 (ampoules) cm3/dose (in secondary packaging)