Product overview
Vaccine type
Hepatitis B
Commercial Name
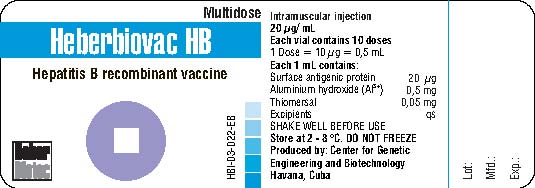
Heberbiovac HB
Manufacturer
Centro de Ingenieria Genetica y Biotecnologia
Responsible NRA
Centro para el Control Estatal de la Calidad de los Medicamentos
Country
Cuba
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
10
Diluent
Not Applicable
Route of Administration
Intramuscular
Shelf Life
48 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 30
Secondary Packaging
1
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be kept for use in subsequent immunization sessions (up to a maximum of 28 days) provided the conditions outlined in the WHO Policy Statement: The use of opened multi-dose vials of vaccine in subsequent immunization sessions are met.
Vaccine Preservative
thiomersal
Vaccine Preservative Concentration
0.05 mg/mL
Cold Chain Volume
2.76 cm3/dose (in secondary packaging)