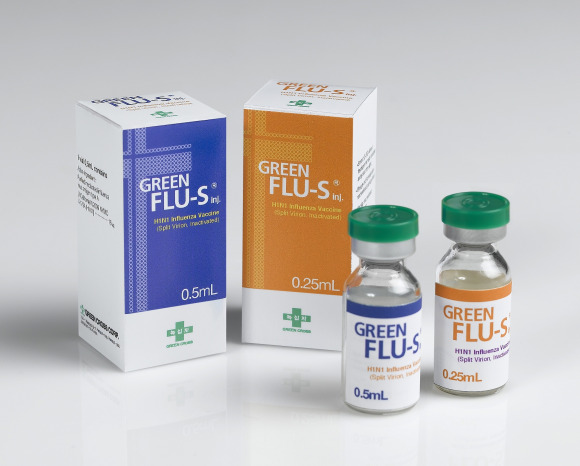
Product overview
Vaccine type
Influenza, Pandemic (H1N1)
Commercial Name
Green Flu-S
Manufacturer
GC Biopharma Corp.
Manufacturer Country
Republic of Korea
Responsible NRA
Ministry of Food and Drug Safety
Country
Republic of Korea
Prequalification
Prequalification Status
Current: No Routine Production
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
1
Diluent
Not Applicable
Route of Administration
Intramuscular
Shelf Life
12 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
none
Secondary Packaging
Single dose carton [Dimensions: 2.5 x 2.5 x 5.5 cm]; Transparent plastic bag containing 10 singles packages.
Tertiary Packaging
Box of 80 plastic bags (800 doses) [Dimensions 76.4 x 59 x 51.5 cm]
Multidose Vial Policy
Not Applicable.
Vaccine Preservative
none
Cold Chain Volume
34.37 cm3/dose (in secondary packaging)