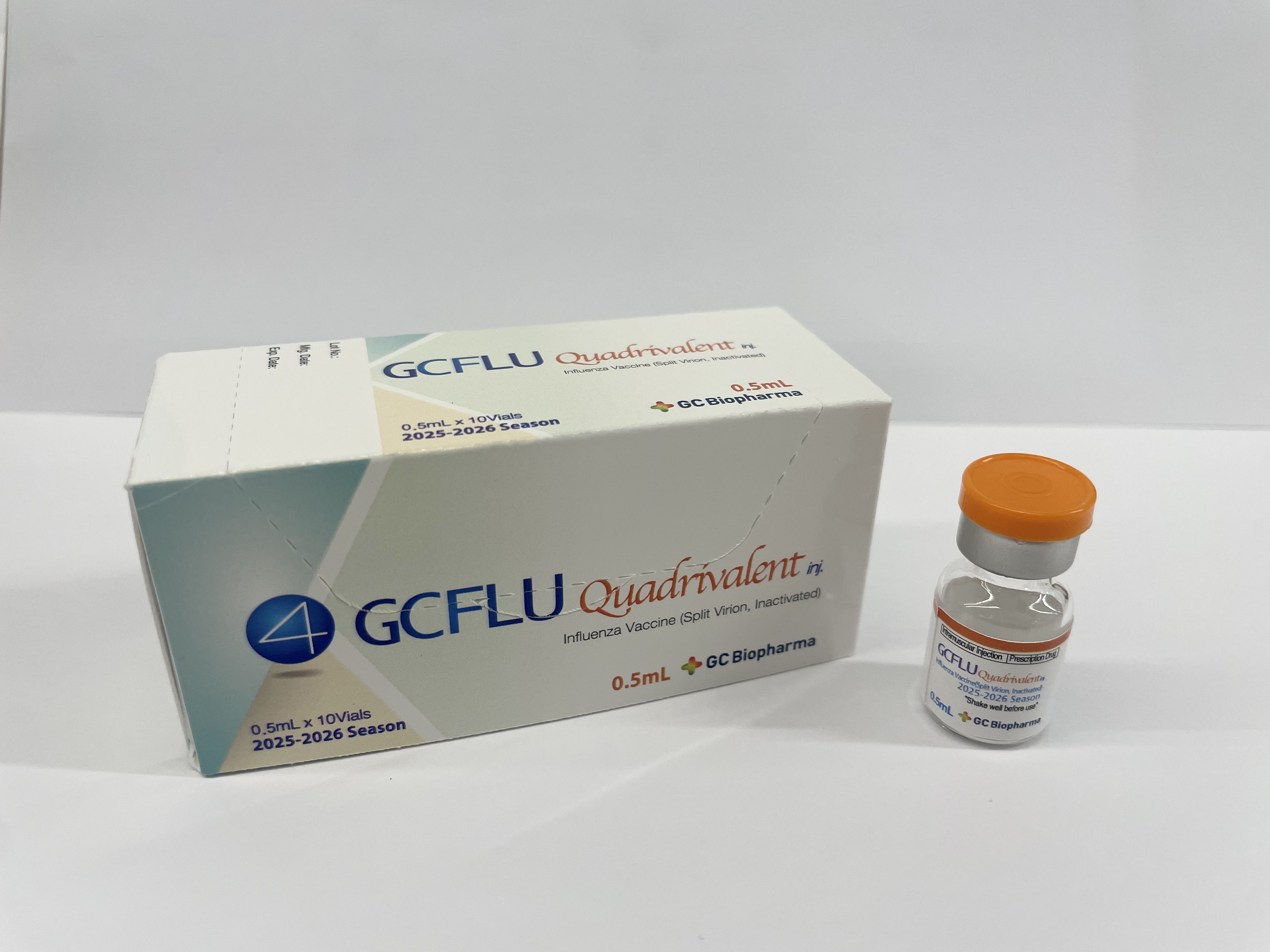
Product overview
Vaccine type
Influenza, seasonal (Quadrivalent)
Commercial Name
GC FLU Quadrivalent inj.
Manufacturer
GC Biopharma Corp.
Manufacturer Country
Republic of Korea
Responsible NRA
Ministry of Food and Drug Safety
Country
Republic of Korea
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
1
Diluent
Not Applicable
Route of Administration
Intramuscular
Shelf Life
12 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 7
Secondary Packaging
a)Single dose carton [Dimensions: 2.5 x 2.5 x 4.5 cm]; b) Carton of 10 vials (10 doses) [Dimensions: 8.8 x 3.6 x 4.4 cm]
Tertiary Packaging
Neo V Box of 3000 single doses cartons [Dimensions: 76 x 58 x 51.7 cm]. Va-Q-Pal of 67200 vials (67200 doses) [Dimensions: 147 x 124 x 158 cm]
Multidose Vial Policy
Not Applicable.
Vaccine Preservative
none
Cold Chain Volume
a)34.37 b)13.94 cm3/dose (in secondary packaging)