Product overview
Vaccine type
cholera: inactivated oral
Commercial Name
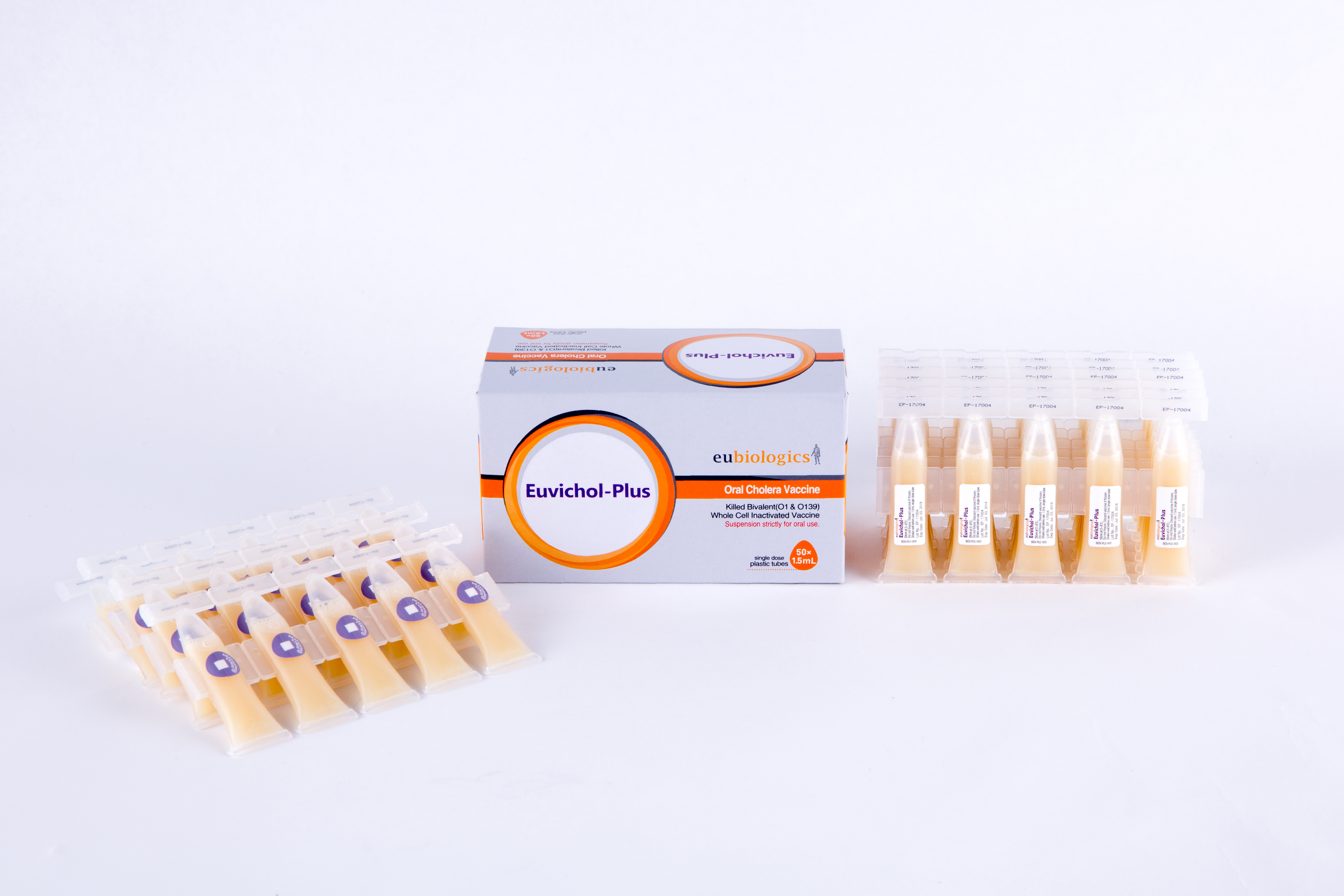
Euvichol-Plus
Manufacturer
EuBiologics Co., Ltd.
Responsible NRA
Ministry of Food and Drug Safety
Country
Republic of Korea
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Plastic Tube
Number of Doses
1
Diluent
Not Applicable
Route of Administration
Oral
Shelf Life
24 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 30
Secondary Packaging
Carton of 10 strips with 5 plastic tubes per strip (50 doses) [Dimensions: 11.1 x 5.7 x 6.2 cm]
Tertiary Packaging
Box [Dimensions: 70 x 70 x 50.5 cm] containing 128 (max.) to 64 (min.) cartons of 50 doses.
Multidose Vial Policy
Not Applicable.
Vaccine Preservative
none
Cold Chain Volume
7.85 cm3/dose (in secondary packaging)
