Product overview
Vaccine type
Hepatitis B
Commercial Name
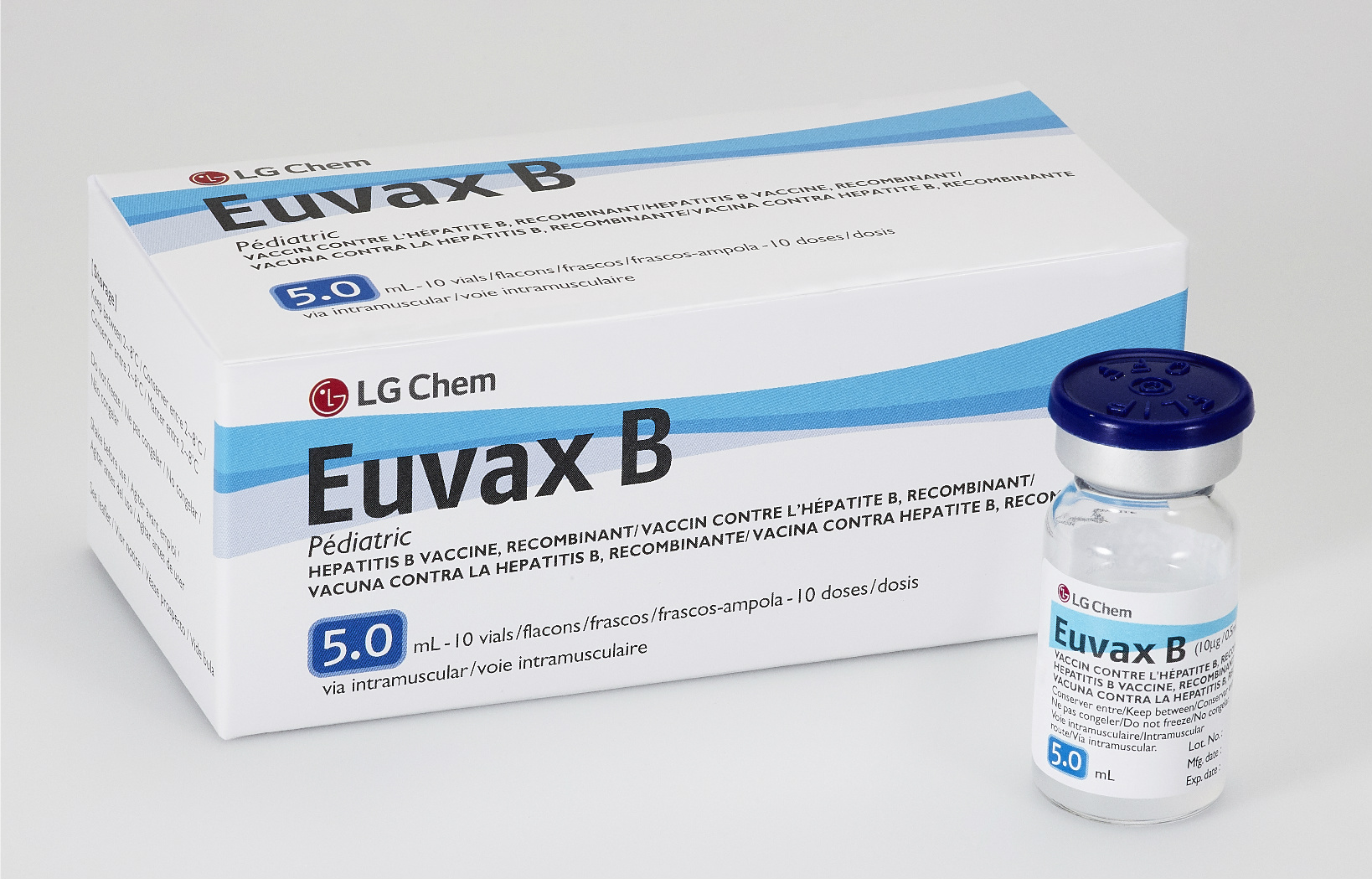
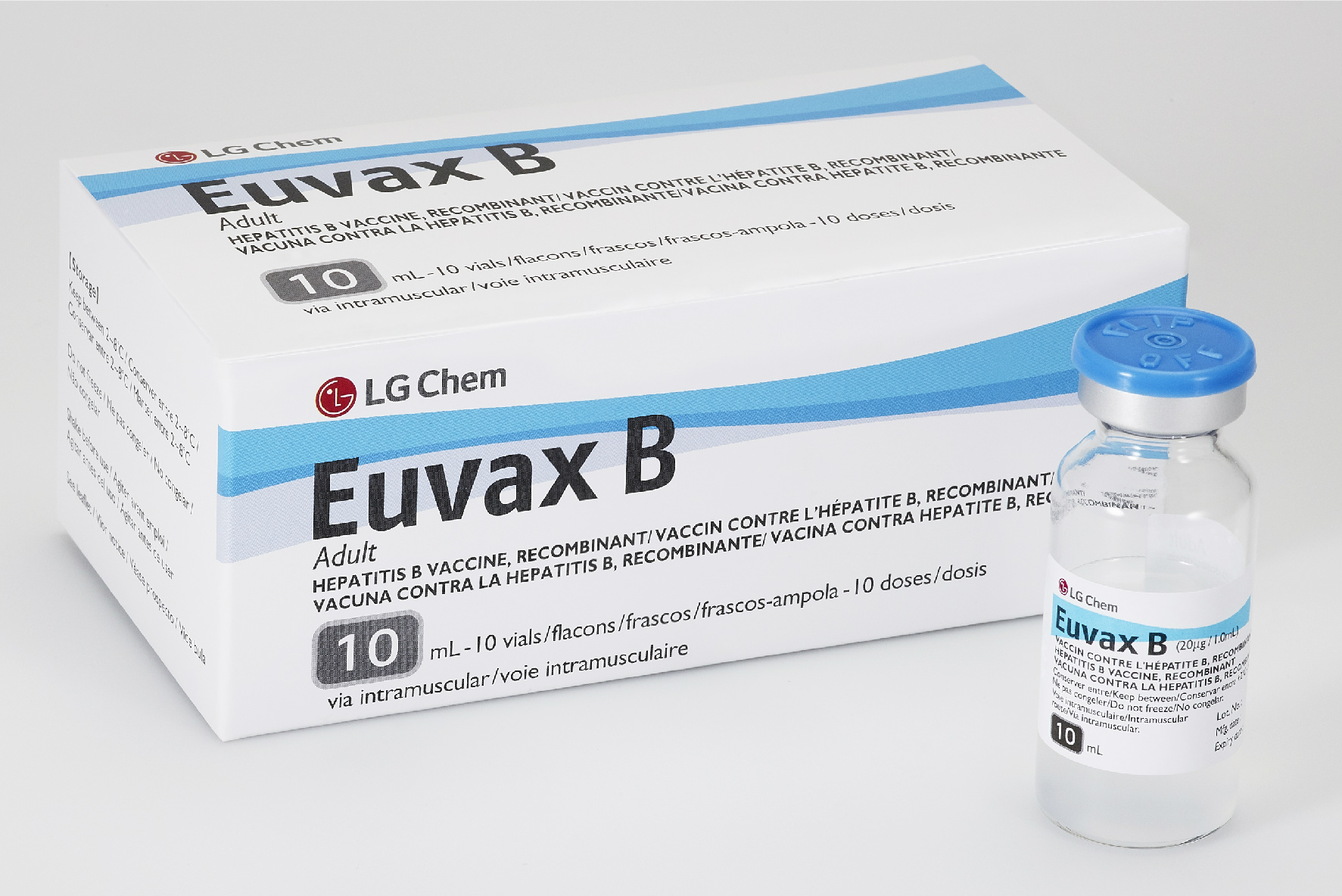
Euvax B
Manufacturer
LG Chem Ltd
Responsible NRA
Ministry of Food and Drug Safety
Country
Republic of Korea
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
10
Diluent
Not Applicable
Route of Administration
Intramuscular
Shelf Life
36 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 30
Secondary Packaging
a)Carton of 10 vials (100 paediatric doses) [Dimensions: 11.5 x 4.7 x 5.3 cm]. b) Carton of 10 vials (100 adult doses) [Dimensions: 13.5x5.4x6.7 cm]
Tertiary Packaging
box of 120 cartons of 10 vials (12,000 doses) [dimensions: 57.5 x 53.5 x 49.0 cm]
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be kept for use in subsequent immunization sessions (up to a maximum of 28 days) provided the conditions outlined in the WHO Policy Statement: The use of opened multi-dose vials of vaccine in subsequent immunization sessions are met.
Vaccine Preservative
thiomersal
Vaccine Preservative Concentration
0.01% w/v
Cold Chain Volume
a) 2.86 paed. b) 4.88 adult cm3/dose (in secondary packaging)