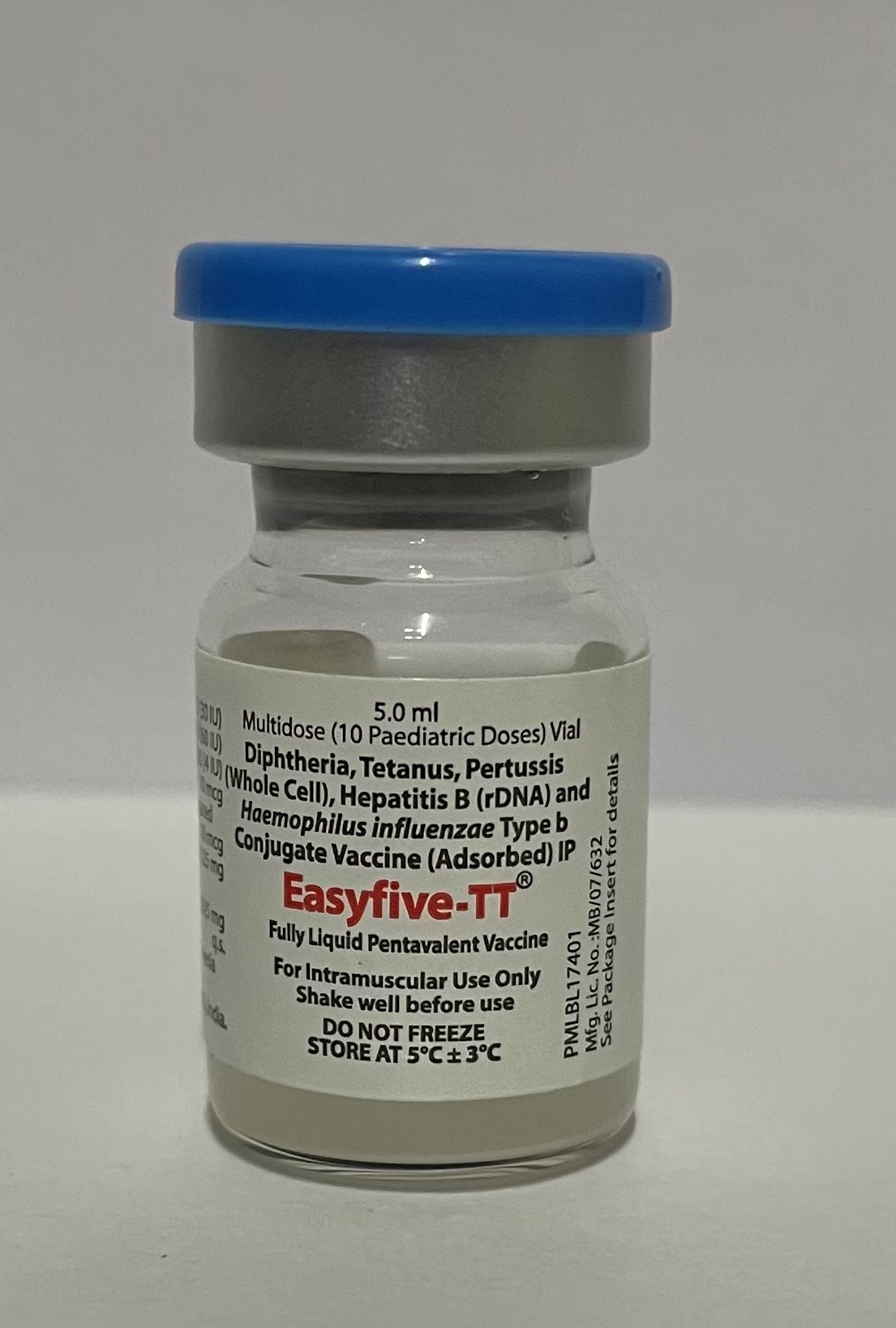
Product overview
Vaccine type
Diphtheria-Tetanus-Pertussis (whole cell)-Hepatitis B-Haemophilus influenzae type b
Commercial Name
Easyfive-TT
Manufacturer
Panacea Biotec Ltd.
Responsible NRA
Central Drugs Standard Control Organization
Country
India
URL
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
10
Diluent
Not Applicable
Route of Administration
Intramuscular
Shelf Life
36 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 14
Secondary Packaging
Carton of 25 vials (250 doses) [dimensions: 13 x 13 x 4.7 cm]
Tertiary Packaging
Shipping box of 24 secondary cartons (6000 doses) [dimensions: 56.9 x 43.9 x 40 cm]
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be kept for use in subsequent immunization sessions (up to a maximum of 28 days) provided the conditions outlined in the WHO Policy Statement: The use of opened multi-dose vials of vaccine in subsequent immunization sessions are met.
Vaccine Preservative
thiomersal
Vaccine Preservative Concentration
0.025 mg/dose
Cold Chain Volume
3.2 cm3/dose (in secondary packaging)