Product overview
Vaccine type
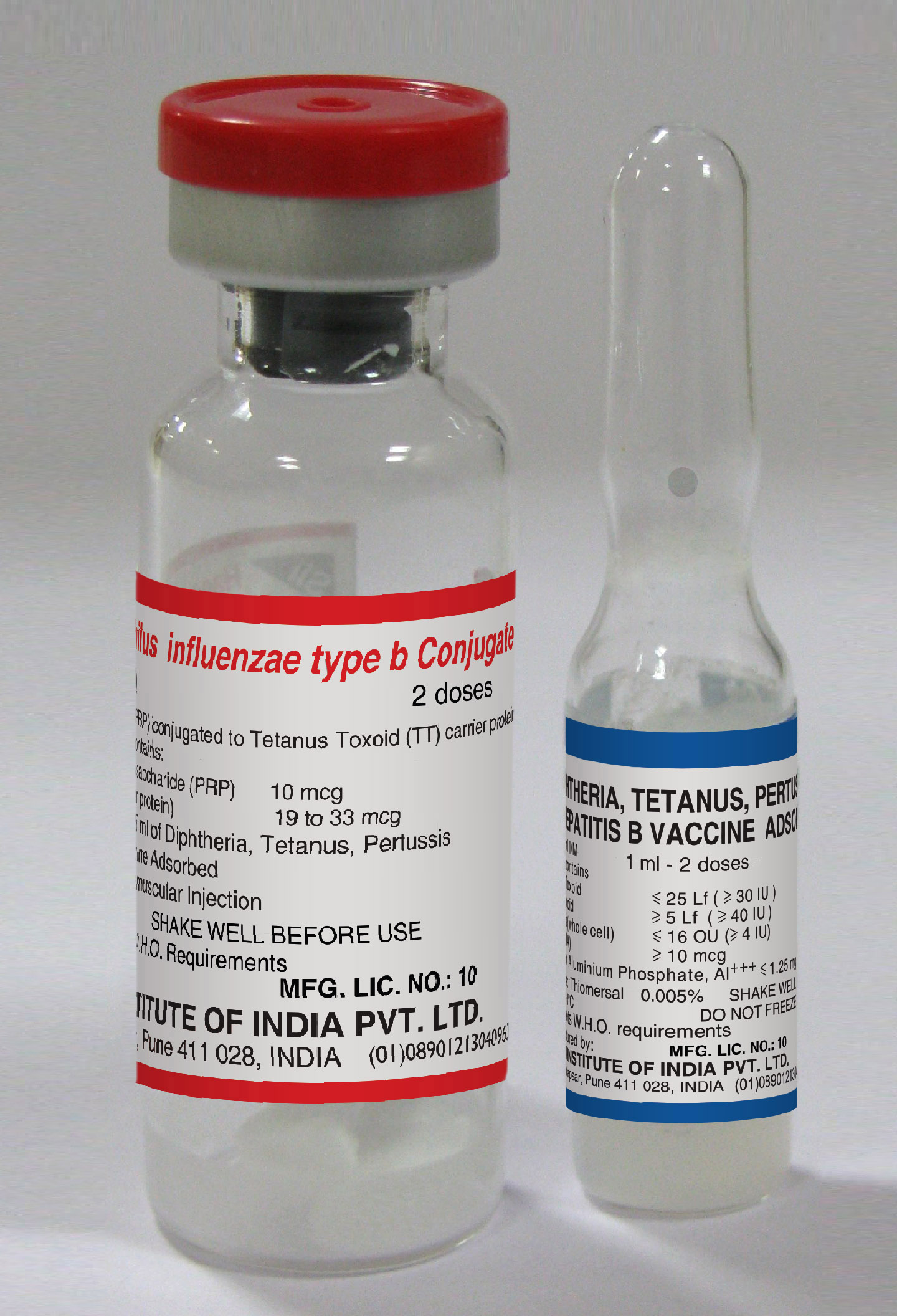
Diphtheria-Tetanus-Pertussis (whole cell)-Hepatitis B-Haemophilus influenzae type b
Commercial Name
Diphtheria, Tetanus, Pertussis, Hepatitis B and Haemophilus influenzae type b Conjugate Vaccine
Manufacturer
Serum Institute of India Pvt. Ltd.
Responsible NRA
Central Drugs Standard Control Organization
Country
India
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Lyophilised active component to be reconstituted with liquid active component before use
Presentation
Vial + Ampoule
Number of Doses
2
Diluent
Liquid active component
Route of Administration
Intramuscular
Shelf Life
24 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 14
Secondary Packaging
a. Carton (containing 4x50 = 200 vials of Hib component [Dimensions 18.5x9.5x6.0 cm] and 4x50= 200 ampoules of DTPwHepB component [Dimensions 14.5x6.0x7.0 cm)
Tertiary Packaging
Box containing 16 cartons of 50 vials of Hib component and 16 cartons of 50 ampoules of DTPwHepB component [Dimensions: 60 x 48 x 41 cm]
Multidose Vial Policy
WHO recommends that opened vials of this vaccine should be discarded 6 hours after opening or at the end of the immunization session, whichever comes first.
Vaccine Preservative
thiomersal
Vaccine Preservative Concentration
0.005%
Cold Chain Volume
a. 10.545(vial); b. 0.609(ampoule) cm3/dose (in secondary packaging)