Product overview
Vaccine type
Diphtheria-Tetanus
Commercial Name
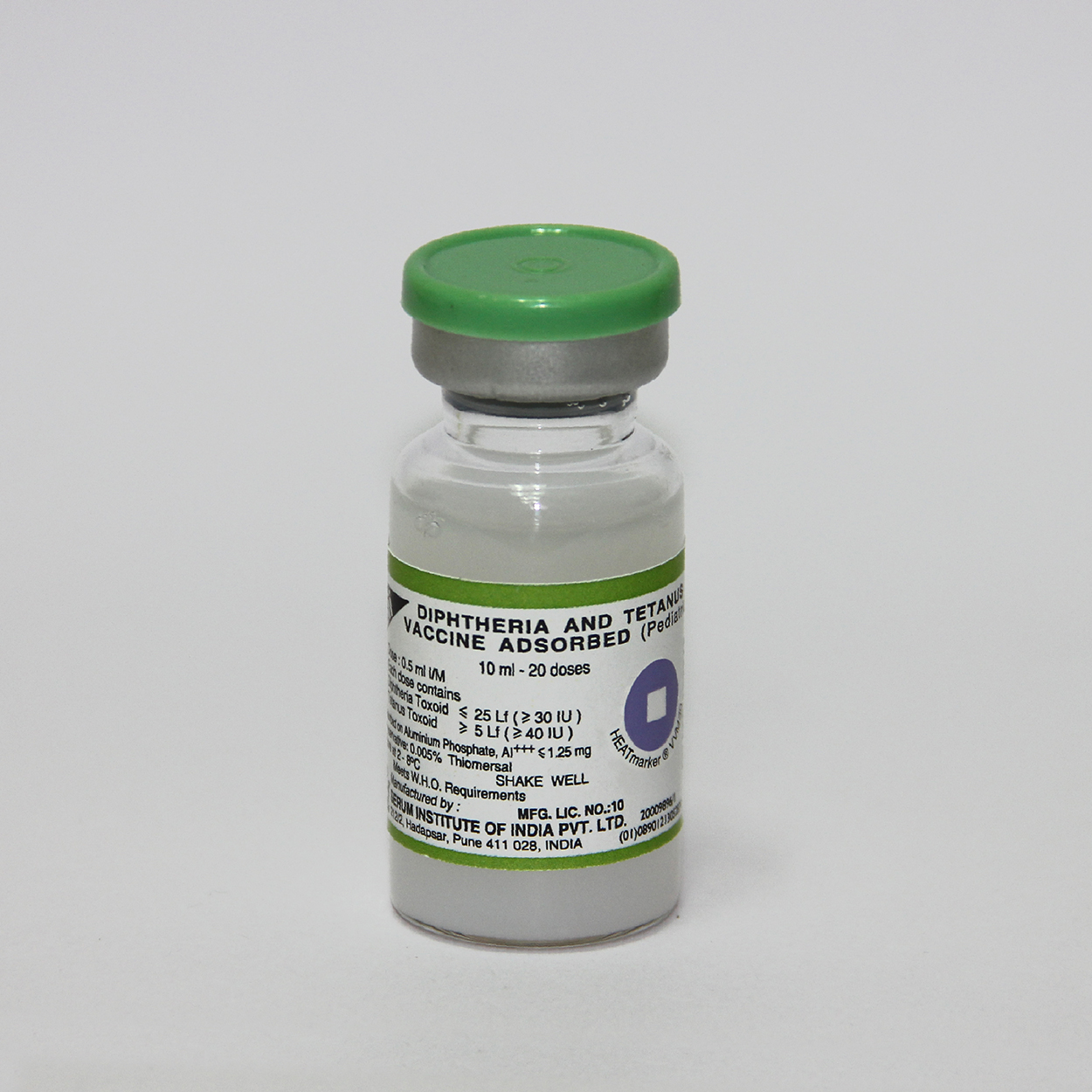
Diphtheria and Tetanus Vaccine Adsorbed (Pediatric)
Manufacturer
Serum Institute of India Pvt. Ltd.
Responsible NRA
Central Drugs Standard Control Organization
Country
India
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
20
Diluent
Not Applicable
Route of Administration
Intramuscular
Shelf Life
36 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
Type 30
Secondary Packaging
Carton of 25 vials (500 doses) [Dimensions 13.3x13.3x6.0 cm]
Tertiary Packaging
Box of 24 cartons of 25 vials (600 vials/12000 doses) [Dimensions 60x48x41 cm]
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be kept for use in subsequent immunization sessions (up to a maximum of 28 days) provided the conditions outlined in the WHO Policy Statement: The use of opened multi-dose vials of vaccine in subsequent immunization sessions are met.
Vaccine Preservative
thiomersal
Vaccine Preservative Concentration
0.005%
Cold Chain Volume
2.122 cm3/dose (in secondary packaging)