Product overview
Vaccine type
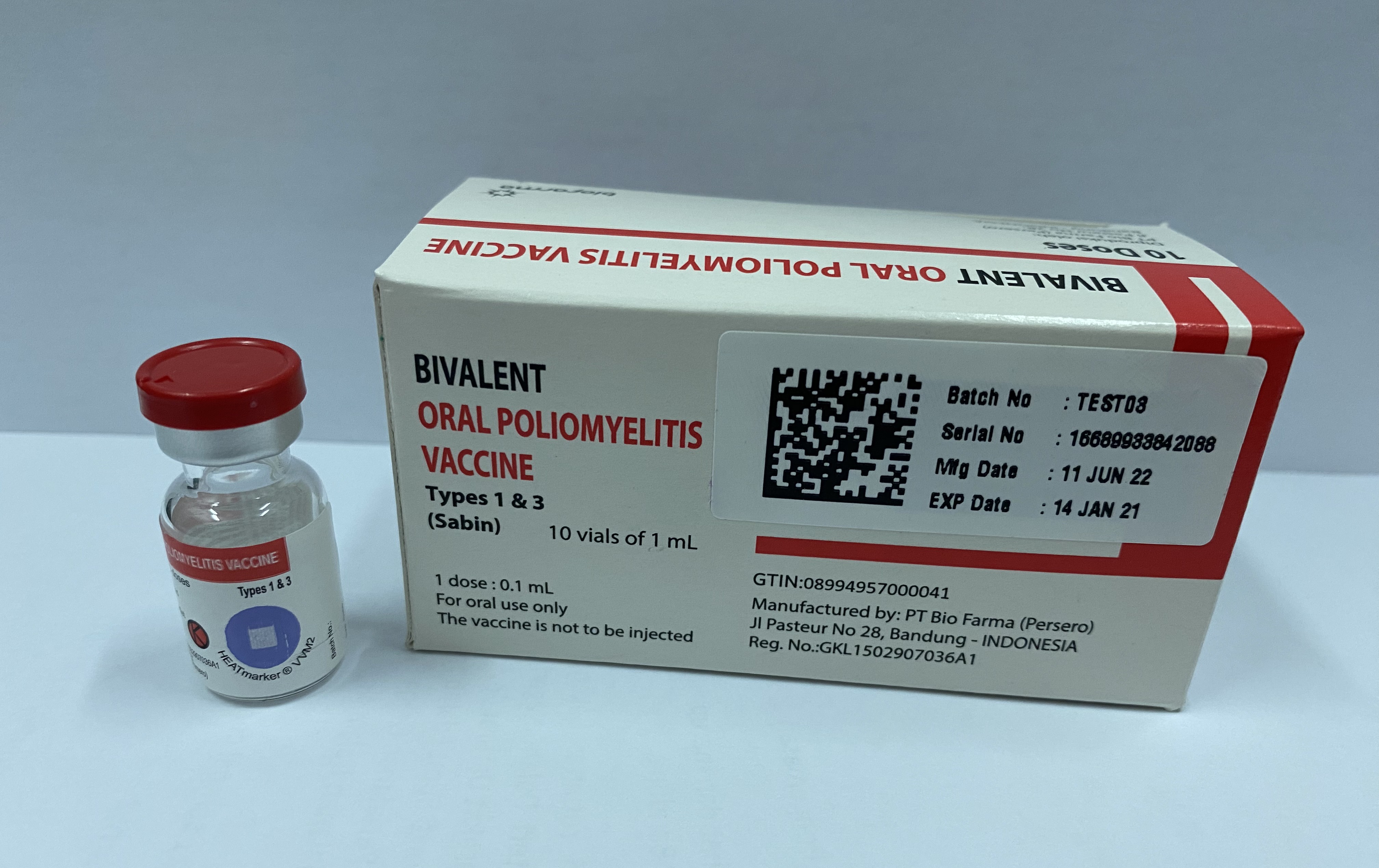
Polio Vaccine - Oral (OPV) Bivalent Types 1 and 3
Commercial Name
Bivalent Oral Poliomyelitis Vaccine Type 1&3 (bOPV 1&3)
Manufacturer
PT Bio Farma (Persero)
Responsible NRA
National Agency of Drug and Food Control Indonesia
Country
Indonesia
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
10
Diluent
Not Applicable
Route of Administration
Oral
Shelf Life
24 months
Storage Temperature
-20°C
Vaccine Vial Monitor
Type 2
Secondary Packaging
carton of 10 vials (8.5 x 3.85 x 4.25 cm)
Tertiary Packaging
Insulated shipping box of 65 x 51 x 60 cm, containing 2700 vials (with icepack) or 3200 vials (with dry ice)
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be kept for use in subsequent immunization sessions (up to a maximum of 28 days) provided the conditions outlined in the WHO Policy Statement: The use of opened multi-dose vials of vaccine in subsequent immunization sessions are met.
Vaccine Preservative
none
Cold Chain Volume
1.4 cm3/dose (in secondary packaging)
