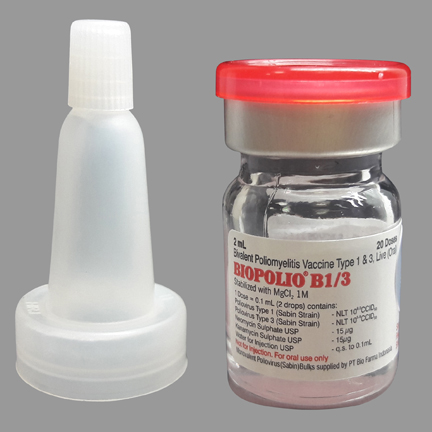
Product overview
Vaccine type
Polio Vaccine - Oral (OPV) Bivalent Types 1 and 3
Commercial Name
BIOPOLIO B1/3
Manufacturer
Bharat Biotech International Limited
Responsible NRA
Central Drugs Standard Control Organization
Country
India
URL
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
20
Diluent
Not Applicable
Route of Administration
Oral
Shelf Life
24 months
Storage Temperature
-20°C
Vaccine Vial Monitor
Type 2
Secondary Packaging
Carton of 25 vials (500 doses) [Dimensions: 11.0 x 11.0 x 3.5 cm]
Tertiary Packaging
Box of 40 cartons of 25 vials (ie. 20,000 doses) [Dimensions:58.5 x 47.5 x 39 cm]
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be kept for use in subsequent immunization sessions (up to a maximum of 28 days) provided the conditions outlined in the WHO Policy Statement: The use of opened multi-dose vials of vaccine in subsequent immunization sessions are met.
Vaccine Preservative
none
Cold Chain Volume
0.85 cm3/dose (in secondary packaging)