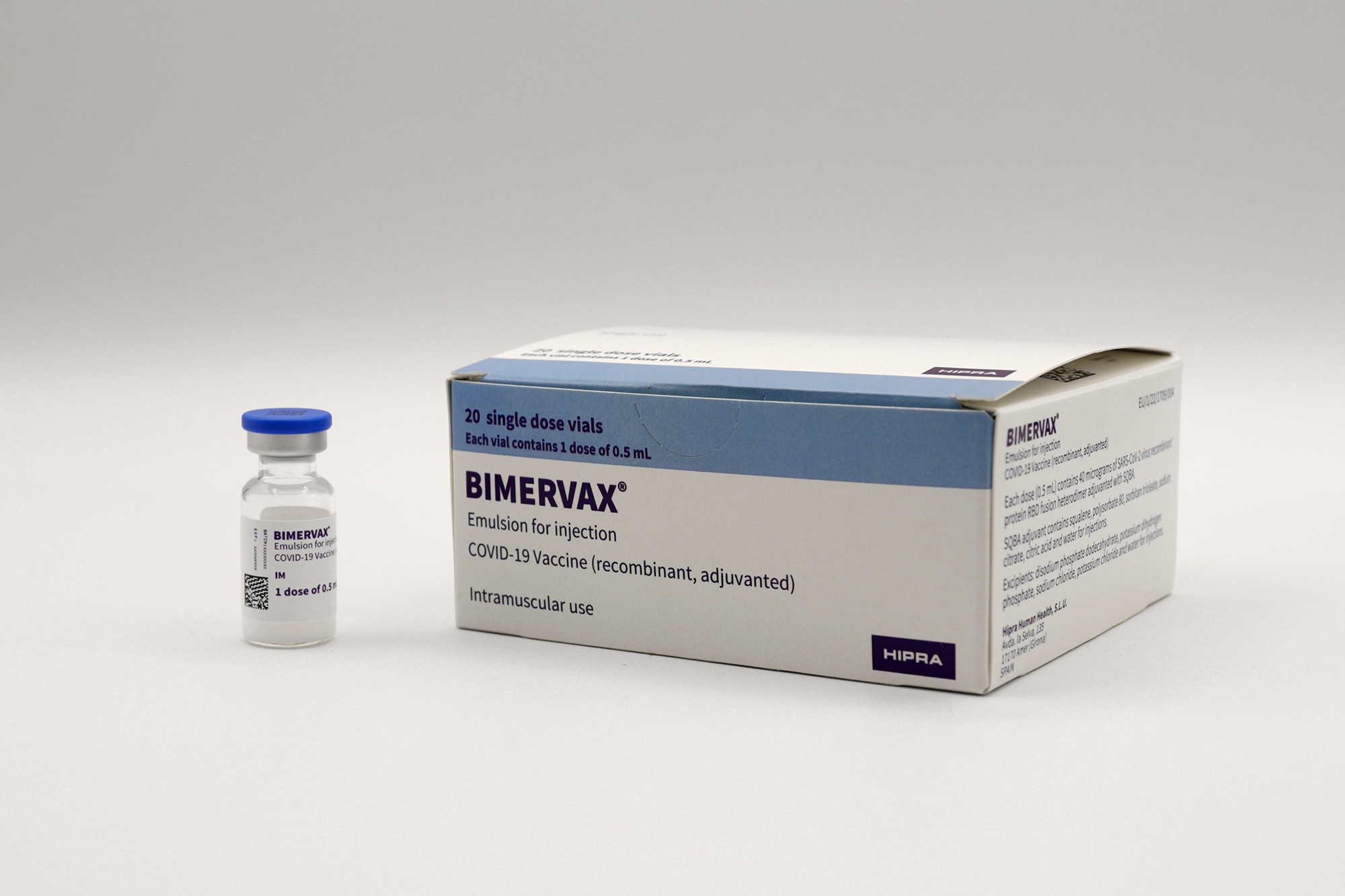
Product overview
Vaccine type
Covid-19
Commercial Name
BIMERVAX
Manufacturer
HIPRA HUMAN HEALTH, S.L.U
Manufacturer Country
Spain
Responsible NRA
European Medicines Agency
Country
Netherlands
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Liquid: ready to use
Presentation
Vial
Number of Doses
1
Diluent
Not Applicable
Route of Administration
Intramuscular
Shelf Life
Omicron (21 months), XBB1.16 and LP.8.1 (12 months)
Storage Temperature
2-8°C and protected from light
Vaccine Vial Monitor
none
Secondary Packaging
To see secondary packaging configuration please refer to the attachment section
Tertiary Packaging
To see tertiary packaging configuration please refer to the attachment section
Multidose Vial Policy
Not applicable