Product overview
Vaccine type
Haemophilus influenzae type b
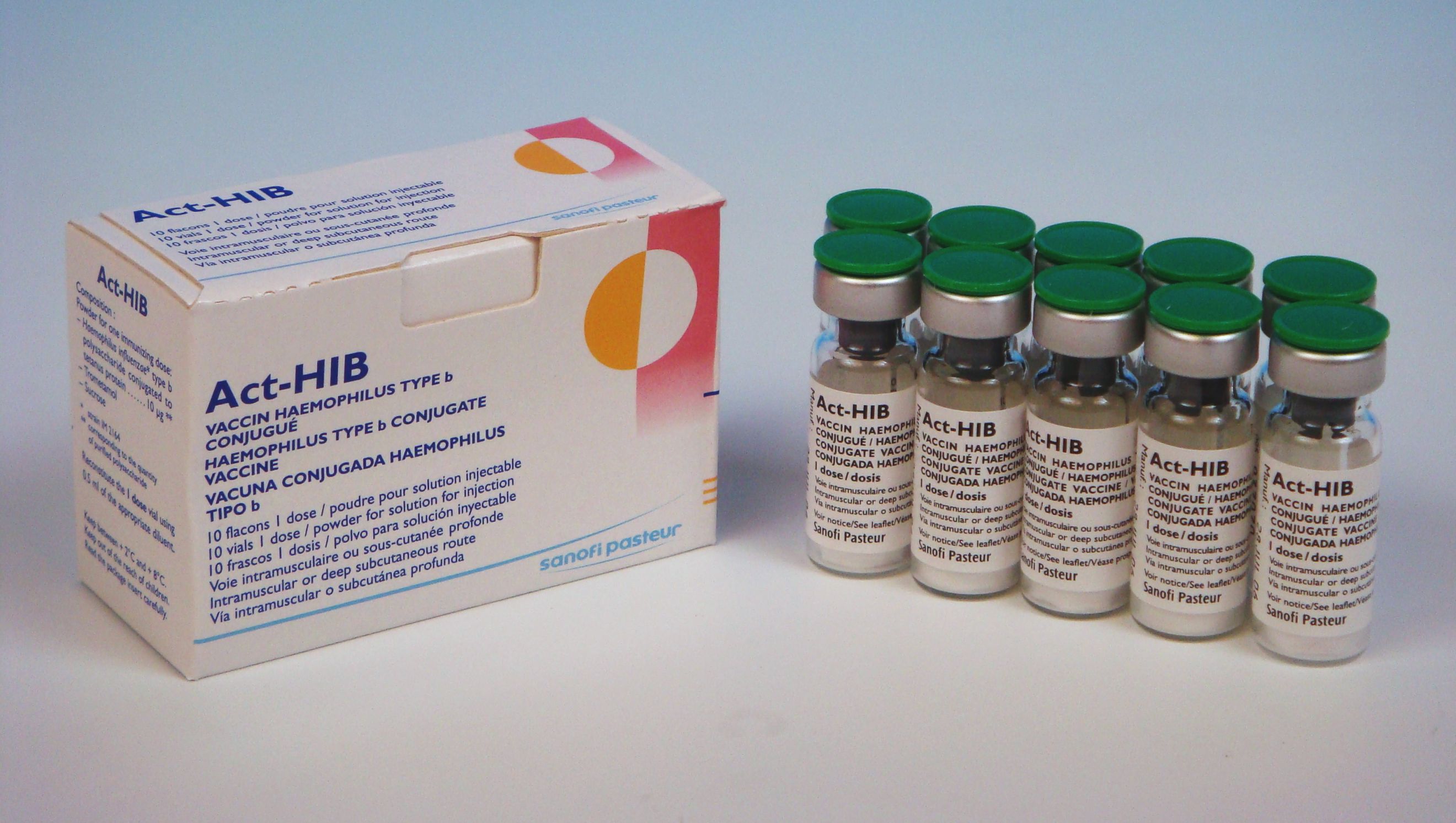
Commercial Name
Act-HIB
Manufacturer
Sanofi Winthrop Industrie
Responsible NRA
Agence nationale de sécurité du médicament et des produits de santé
Country
France
Prequalification
Prequalification Status
Current
Product description
Pharmaceutical Form
Lyophilised active component to be reconstituted with excipient diluent before use
Presentation
Vial
Number of Doses
1
Diluent
0.4 % Sodium Chloride in ampoule
Route of Administration
Intramuscular or Subcutaneous
Shelf Life
36 months
Storage Temperature
2-8°C
Vaccine Vial Monitor
none
Secondary Packaging
10 vials 1 dose (vaccine)
Multidose Vial Policy
Not Applicable.
Vaccine Preservative
none
Cold Chain Volume
12.66 cm3/dose (in secondary packaging)