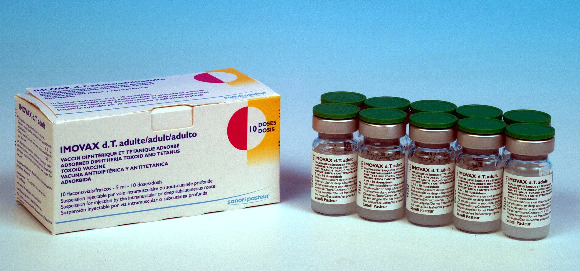
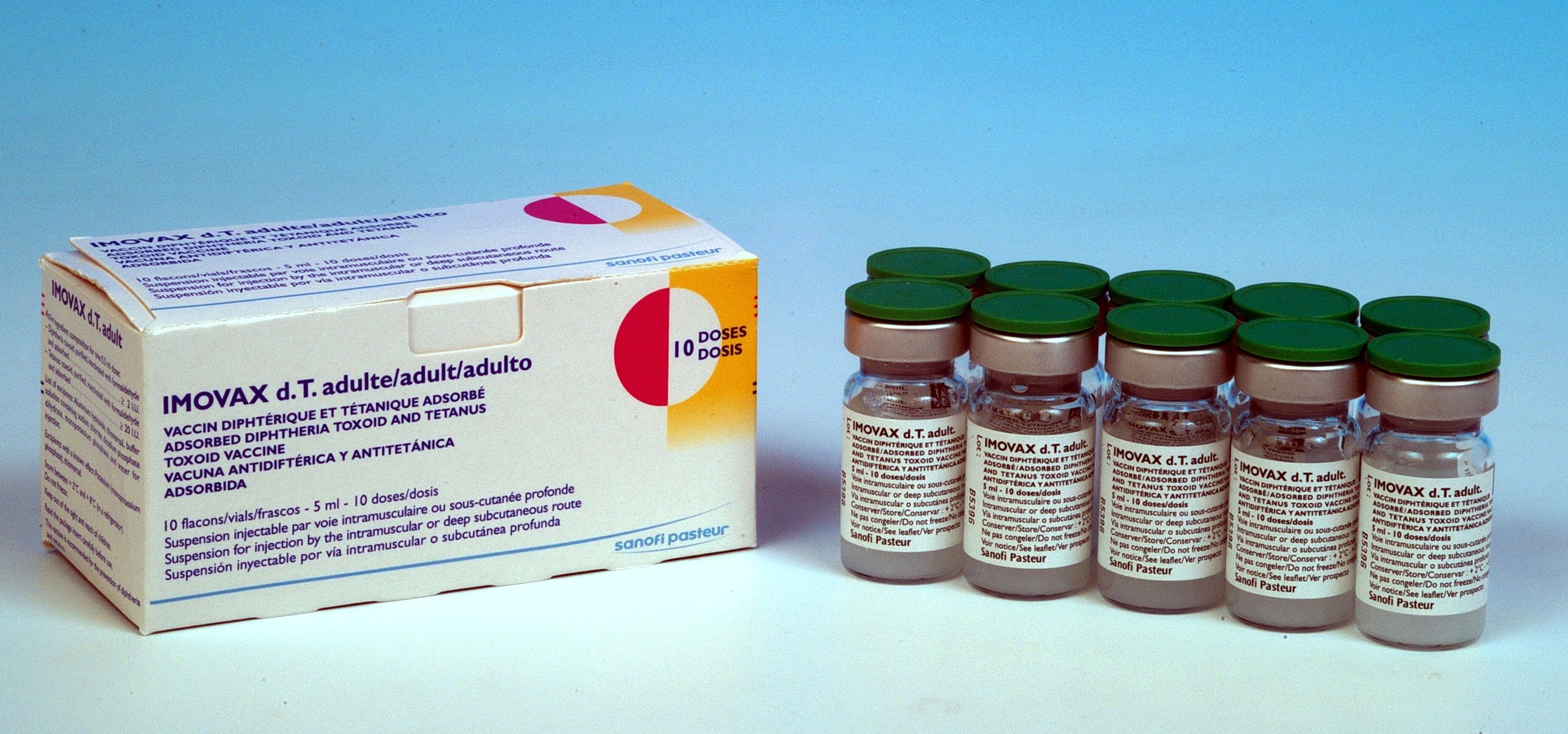
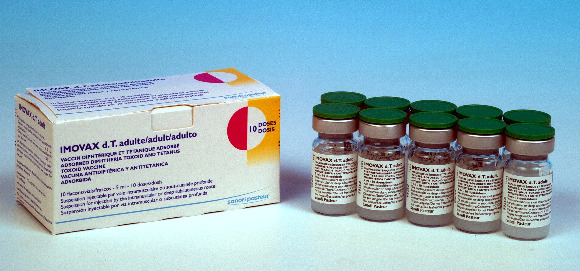
IMOVAX dT adult
Diphtheria and tetanus vaccine (adsorbed, reduced diphtheria antigen content)
Product Details
WHO Product ID
FVP-P-92
Status
Prequalified
Date of prequalification
Vaccine type
Diphtheria and tetanus vaccine (adsorbed, reduced diphtheria antigen content)
Abbreviated Name
dT
Commercial Name
IMOVAX dT adult
PQ Holder
Sanofi Pasteur SA
14 Espace Henry Vallée
Lyon,
69007
France
Responsible NRA
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
Product description
Pharmaceutical Form
Liquid: Ready to use
Presentation
Vial
Number of doses
10
Route of administration
Intramuscular
Vaccine Vial Monitor
None
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be used in subsequent immunization sessions (up to 28 days) if the conditions outlined in the WHO Policy Statement are met.
Shelf life (months)
36 months
Storage temperature
2 - 8°C
Diluent
N/A
Preservative
Thiomersal
Preservative concentration
0.01%
Product Packaging
Secondary Container
Component Packed
Active (Vaccine)
Total doses
10
Description
Carton of 10 vials (100 doses)[Dimensions: 11 x 4.7 x 4.9 cm]
Dimensions
11.0 x 4.7 x 4.9 cm
Cold Chain Volume (cm³/dose)
2.53
Attachments