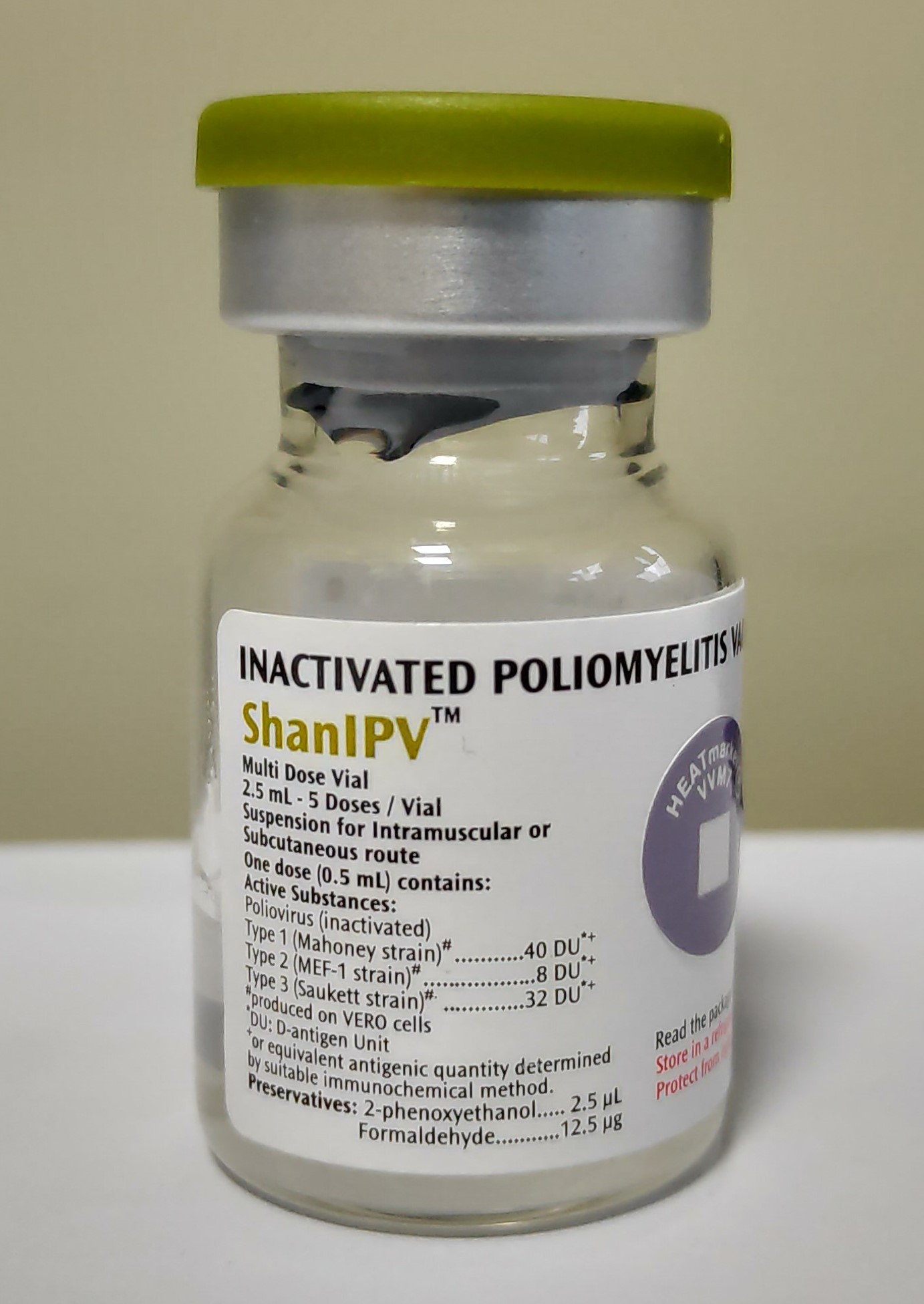
ShanIPV™
Poliomyelitis vaccine (inactivated)
Product Details
WHO Product ID
FVP-P-333
Status
Prequalified
Date of prequalification
Vaccine type
Poliomyelitis vaccine (inactivated)
Abbreviated Name
IPV
Commercial Name
ShanIPV™
PQ Holder
Sanofi Healthcare India Private Limited
Sanofi house CTS-117B L&T Bussiness Park Saki Vihar Road Powai
Mumbai,
Maharashtra
400072
India
Responsible NRA
Central Drugs Standard Control Organization (CDSCO)
Product description
Bulk supplier
Sanofi Pasteur SA
Pharmaceutical Form
Liquid: Ready to use
Presentation
Vial
Number of doses
5
Route of administration
Intramuscular or subcutaneous
Vaccine Vial Monitor
Type 11
Multidose Vial Policy
WHO recommends that opened vials of this vaccine may be used in subsequent immunization sessions (up to 28 days) if the conditions outlined in the WHO Policy Statement are met.
Shelf life (months)
36 months
Storage temperature
2 - 8°C
Diluent
N/A
Preservative
2-phenoxyethanol
Preservative concentration
2.5 µL/dose
Remarks
Formaldehyde with concentration 12.5 µg/dose is used as preservative in combination with 2-Phenoxyethanol .VVM 11 will reach its end point after 30 months at 2°C to 8°C while the vaccines is still within its shelf-life. In such case the vaccine vials in question should be discarded.
Product Packaging
Secondary Container
Tertiary Container
Component Packed
Active (Vaccine)
Total doses
30
Description
Carton of 30 vials (150 doses) [Dimensions: 4.9 x 12.5 x 14.9 cm].
Dimensions
4.9 x 12.5 x 14.9 cm
Cold Chain Volume (cm³/dose)
6.08