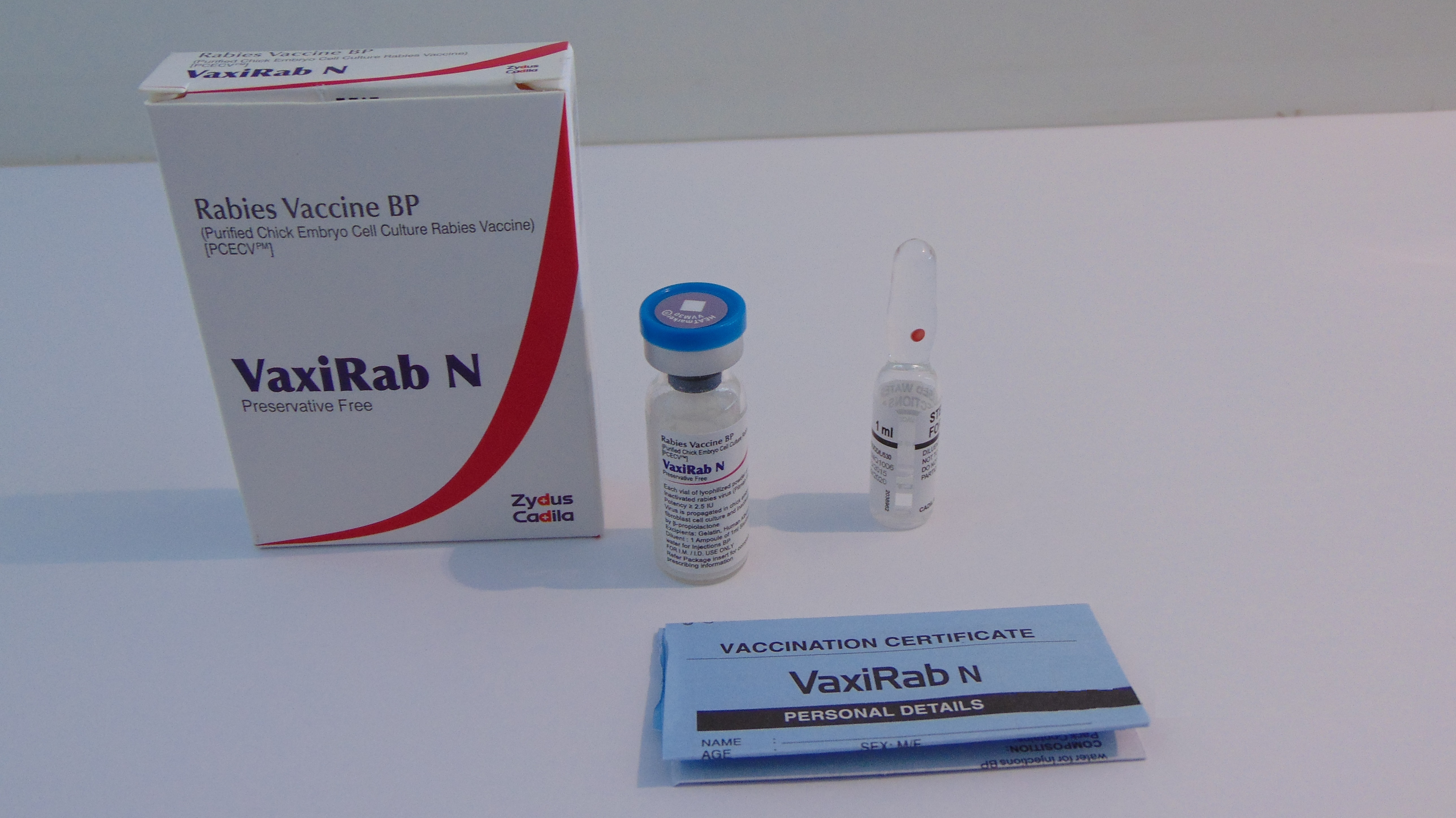
VaxiRab N
Rabies vaccine for human use
Product Details
WHO Product ID
FVP-P-330
Status
Prequalified
Vaccine type
Rabies vaccine for human use
Abbreviated Name
Rabies
Commercial Name
VaxiRab N
PQ Holder
Zydus Lifescience Limited
Survey No. 536 Khoraj (Gandhinagar), Near Vaishanodevi Circle
Ahmedabad,
Gujarat
382481
India
Responsible NRA
Central Drugs Standard Control Organization (CDSCO)
Product description
Pharmaceutical Form
Lyophilised active component to be reconstituted with excipient diluent before use
Presentation
Vial
Number of doses
1
Route of administration
Intramuscular
Vaccine Vial Monitor
Type 30
Multidose Vial Policy
Not applicable
Shelf life (months)
36 months
Storage temperature
2 - 8°C
Diluent
Water for injection
Preservative
None
Remarks
VaxiRab N can also be administered intradermally. Please refer to the Product Insert (PI).
Product Packaging
Secondary Container
Tertiary Container
Component Packed
Active (Vaccine)
Total doses
10
Description
Active: Carton of 10 single dose vials (10 doses) [Dimensions: 2.3 x 2.3 x 5.5 cm]
Dimensions
2.3 x 2.3 x 5.5 cm
Cold Chain Volume (cm³/dose)
2.91
Component Packed
Diluent
Total doses
10
Description
Diluent:Carton of 50 ampoules (50 doses)[Dimensions: 27.5 x 13.2 x 6.6 cm]
Dimensions
27.5 x 13.2 x 6.6 cm
Cold Chain Volume (cm³/dose)
47.92