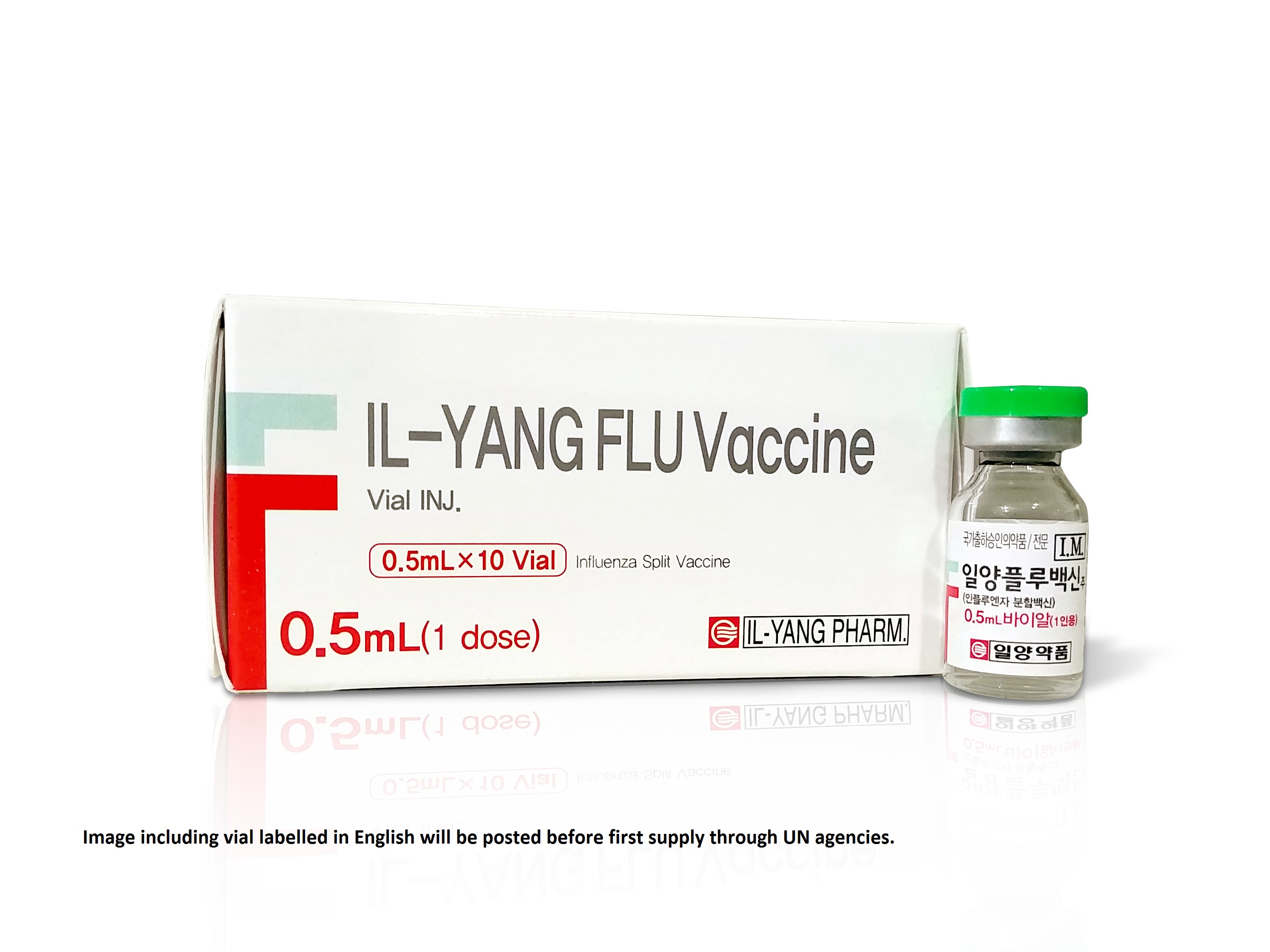
IL-YANG FLU Vaccine INJ.
Influenza vaccine trivalent (split virion, inactivated)
Product Details
WHO Product ID
FVP-P-293
Status
Prequalified
Date of prequalification
Vaccine type
Influenza vaccine trivalent (split virion, inactivated)
Abbreviated Name
IIV3
Commercial Name
IL-YANG FLU Vaccine INJ.
PQ Holder
IL-YANG PHARMACEUTICAL CO. LTD.
110 Hagal-ro Giheung-gu Yongin-si
Gyeonggi-do,
446-726
Republic of Korea
Responsible NRA
Ministry of Food and Drug Safety (MFDS)
Product description
Pharmaceutical Form
Liquid: Ready to use
Presentation
Vial
Number of doses
1
Route of administration
Intramuscular
Vaccine Vial Monitor
Type 2
Multidose Vial Policy
Not applicable
Shelf life (months)
12 months
Storage temperature
2 - 8°C
Diluent
N/A
Preservative
None
Product Packaging
Secondary Container
Tertiary Container
Component Packed
Active (Vaccine)
Total doses
10
Description
Carton of 10 vials (10 doses) [Dimensions: 9.0 x 3.8 x 4.5 cm]
Dimensions
9.0 x 3.8 x 4.5 cm
Cold Chain Volume (cm³/dose)
15.39