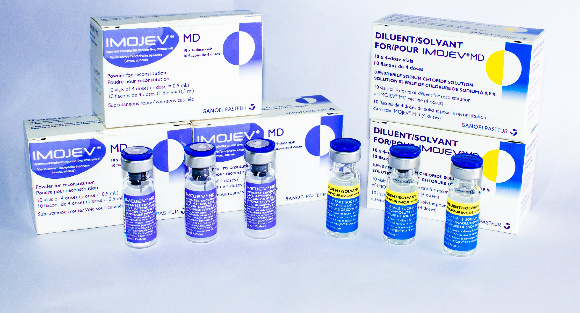
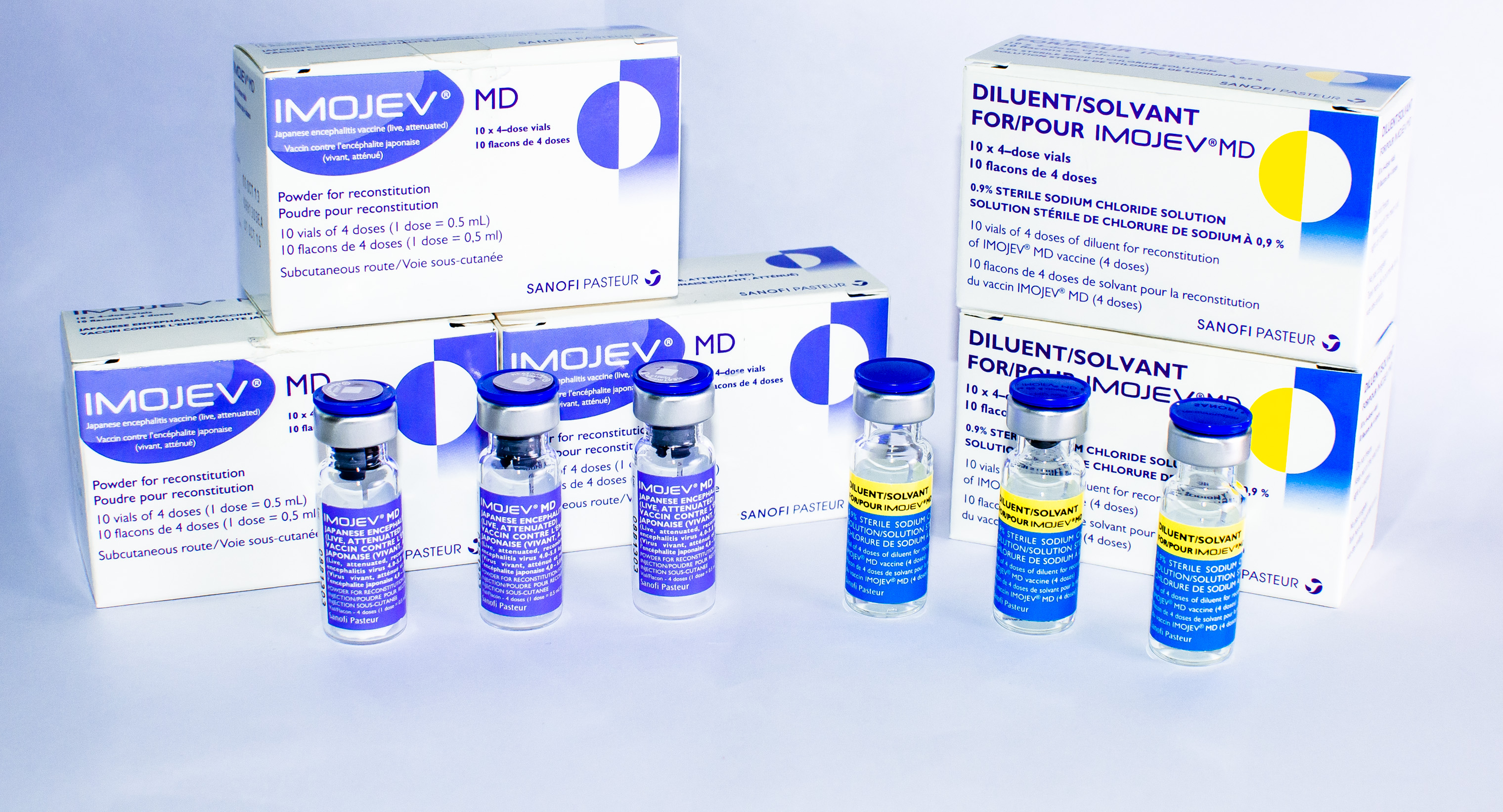
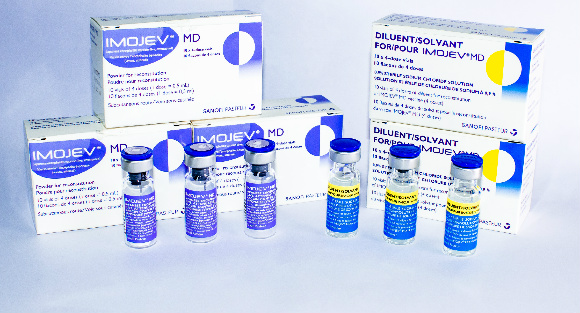
IMOJEV MD
Japanese encephalitis vaccine (live, attenuated) for human use
Product Details
WHO Product ID
FVP-P-276
Status
Prequalified
Date of prequalification
Vaccine type
Japanese encephalitis vaccine (live, attenuated) for human use
Abbreviated Name
JE
Commercial Name
IMOJEV MD
PQ Holder
GPO-MBP Co. Ltd. (GPO-MBP)
241 Moo 7 (Plaengyo Ind. Estate)Tambon Huasamrong, Chachoengsao
Amphoe Plaengyao,
24190
Thailand
Responsible NRA
Thai Food and Drug Administration (FDA)
Product description
Bulk supplier
Sanofi Pasteur Inc.
Pharmaceutical Form
Lyophilised active component to be reconstituted with excipient diluent before use
Presentation
Two vial set (active + excipient)
Number of doses
4
Route of administration
Subcutaneous
Vaccine Vial Monitor
Type 14
Multidose Vial Policy
WHO recommends that opened vials of this vaccine should be discarded 6 hours after opening or at the end of the immunization session, whichever comes first.
Shelf life (months)
36 months
Storage temperature
2 - 8°C
Diluent
Sodium chloride (0.9%)
Preservative
None
Remarks
The manufacturer of the finished product is Government Pharmaceutical Organization-Merieux Biological Products Co (GPO-MBP) a joint venture between GPO (Thailand) and Sanofi Pasteur. Sanofi Pasteur is the owner of the product as indicated in product labelling.
Product Packaging
Secondary Container
Tertiary Container
Shipping Container
Component Packed
Active (Vaccine)
Total doses
10
Description
Carton of 10 vials (active) (40 doses) [Dimensions 7.4 x 3.1 x 4.3 cm]
Dimensions
7.4 x 3.1 x 4.3 cm
Cold Chain Volume (cm³/dose)
2.47
Component Packed
Diluent
Total doses
10
Description
Separate carton of 10 vials (diluent) (which can be stored outside cold chain)
Cold Chain Volume (cm³/dose)
0.0