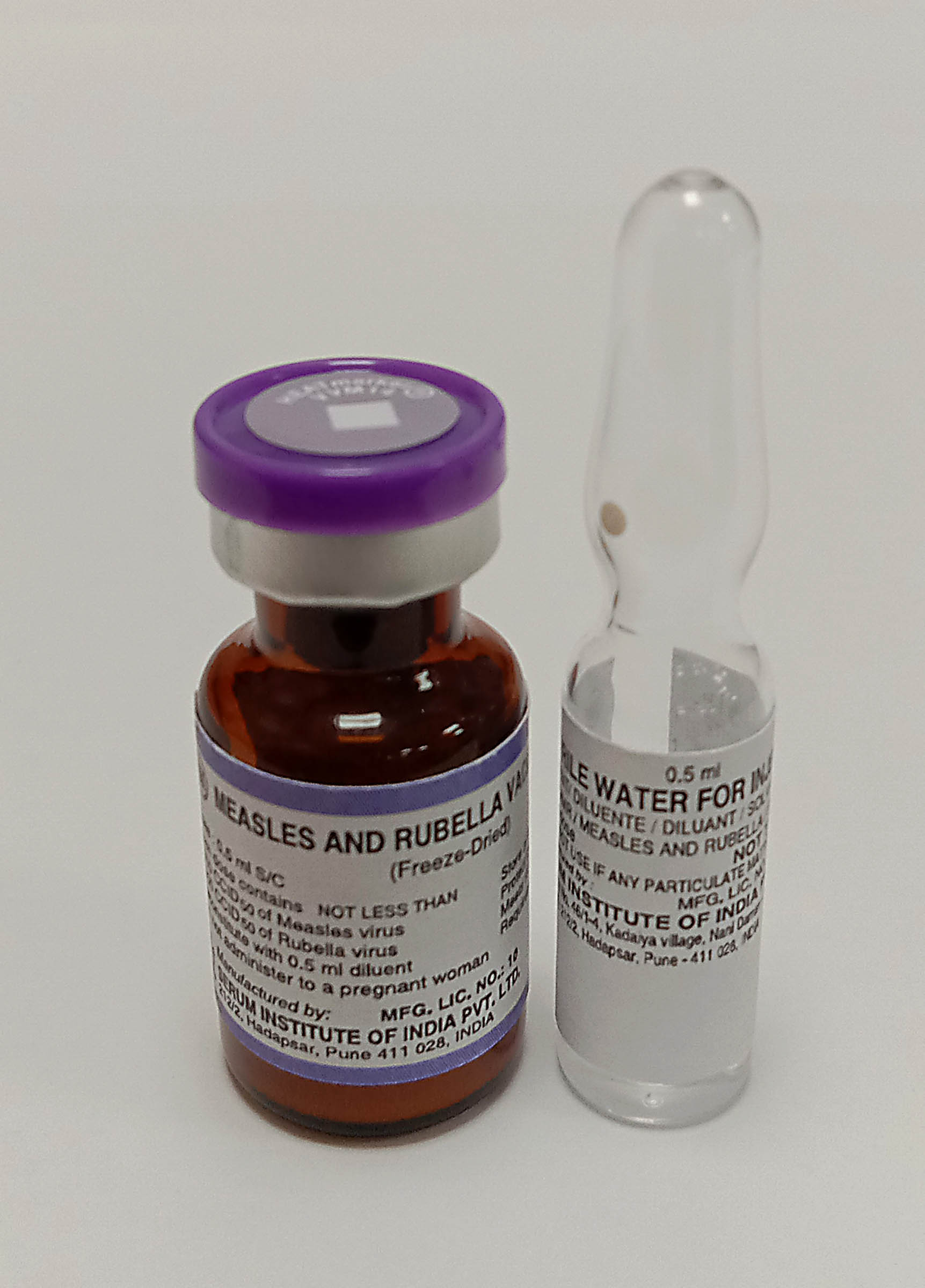
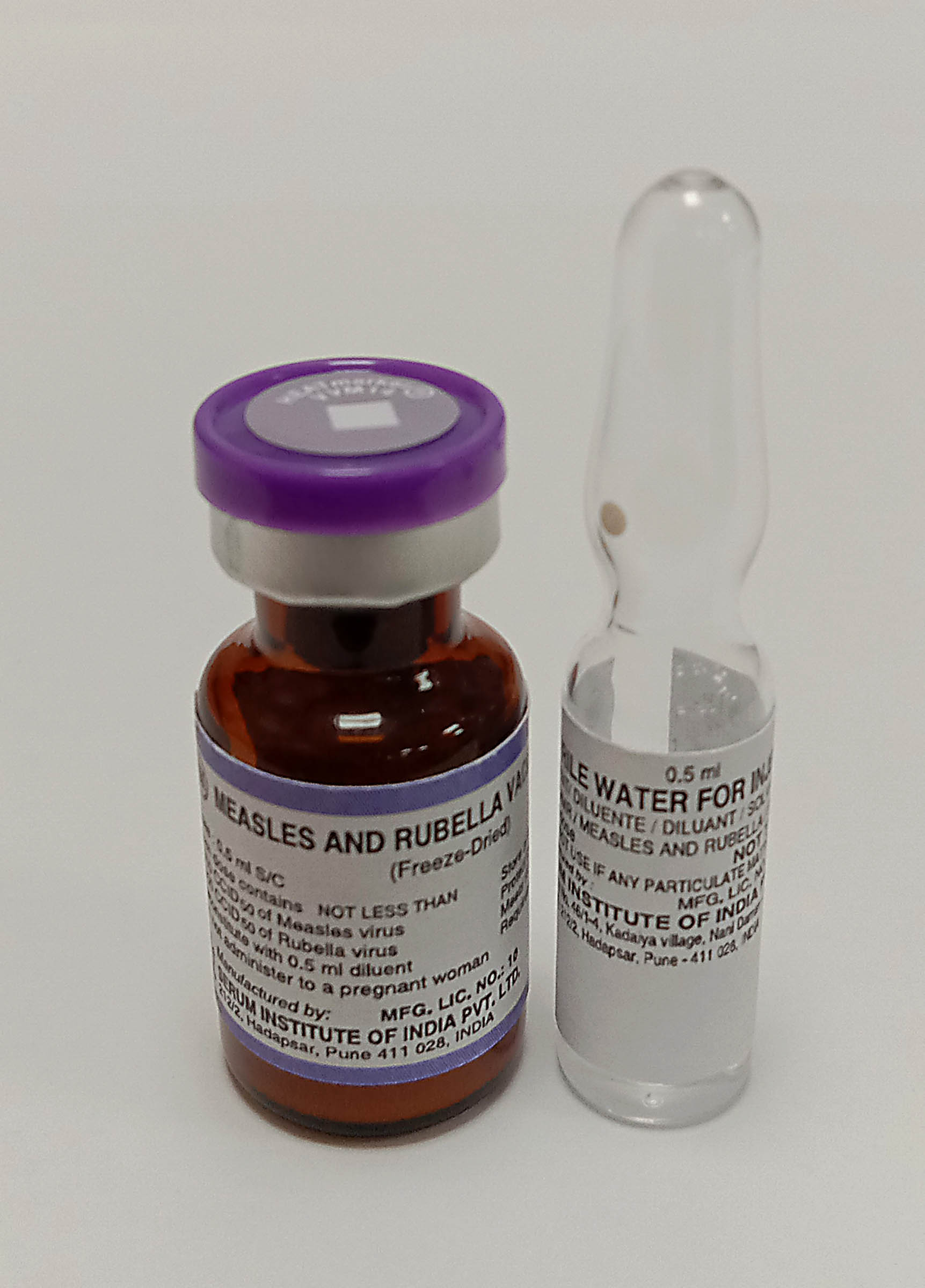
Measles and Rubella Vaccine Live Attenuated
Measles, rubella combined vaccine (live, attenuated)
Product Details
WHO Product ID
FVP-P-137
Status
Prequalified
Date of prequalification
Vaccine type
Measles, rubella combined vaccine (live, attenuated)
Abbreviated Name
MR
Commercial Name
Measles and Rubella Vaccine Live Attenuated
PQ Holder
Serum Institute of India Pvt. Ltd.
212/2 Off Soli Poonawala Road, Hadaspar
Pune,
Maharashtra
411 028
India
Responsible NRA
Central Drugs Standard Control Organization (CDSCO)
Product description
Pharmaceutical Form
Lyophilised active component to be reconstituted with excipient diluent before use
Presentation
Vial
Number of doses
1
Route of administration
Subcutaneous
Vaccine Vial Monitor
Type 14
Multidose Vial Policy
Not applicable
Shelf life (months)
30 months
Storage temperature
2 - 8°C
Diluent
Water for injection
Preservative
None
Remarks
An extension of shelf life at 2-8°C from 24 to 30 months was approved on 10 January 2018 and the Product Insert (PI) indicating this shelf life is part of this web listing. However for some time stock on-hand manufactured before this approval date may be supplied and the PI for these batches will still indicate a 24 month shelf life.
Product Packaging
Secondary Container
Tertiary Container
Component Packed
Active (Vaccine)
Total doses
50
Description
Carton of 50 vials (active)(50 doses) [Dimensions 18.5x9.5x6.0 cm]
Dimensions
18.5 x 9.5 x 6.0 cm
Cold Chain Volume (cm³/dose)
21.09
Component Packed
Diluent
Total doses
50
Description
Carton of 50 ampoules (diluent) [Dimesions 14.5x6.0x7.2 cm]
Dimensions
14.5 x 6.0 x 7.2 cm
Cold Chain Volume (cm³/dose)
12.53